Best Clinical Research Institute
Clinical Research Sub-Courses
CliniLaunch
- 100% Placement Assistance
- IAO Accredited Courses
- Industry-Ready Curriculum
- NSDC Certified Institution
Healthcare Diploma Courses for Pharma & IT Professionals
Free Demo Class Today
Talk to CliniLaunch’s expert counselor. Get Insights on a range of progressive career options today!
Free Demo Class Today
Talk to CliniLaunch’s expert counselor. Get Insights on a range of progressive career options today!
Clinical Research Sub-Courses
CliniLaunch
- 100% Placement Assistance
- IAO Accredited Courses
- Industry-Ready Curriculum
- NSDC Certified Institution
Healthcare Diploma Courses for Pharma & IT Professionals

IAO & NSDC Accredited Courses

LSSSDC Membership

470+ Corporate Partnerships

10,000+ students trained
Clinical Research Sub-Courses in Bangalore
Collect and manage data for your clinical research studies and documentation and learn how to build and capture electronic data instruments.
Modal Box Title
- Courses
- Clinical Data Management
Clinical Data Management Course
Any Question? Happy to Answer
Clinical Research Sub-Courses
- 11k+ Students
A course that can be your gateway to a successful career in clinical research. The importance of efficient clinical data management cannot be overstated because the demand for skilled professionals in this domain continues to rise, and individuals seeking a rewarding career in clinical data management often find themselves in search of the best courses available.
What is Clinical Data Management?
Clinical Data Management (CDM) is a critical discipline in the clinical research process. It ensures the accuracy, completeness, and consistency of clinical trial data. CDMs play a vital role in protecting the rights and safety of study subjects and ensuring the integrity of research data.
Our experienced staff uses our well-documented processes and service levels agreements to ensure timely deliverables. We handle all phases of clinical research across the full spectrum of therapeutic areas. CDM plays a vital role in maintaining data integrity and quality, ultimately contributing to the reliability of conclusions drawn from clinical studies, and facilitating the development of safe and effective medical treatments.
Learnings the program offers
- Clinical Data Management fundamentals.
- Data collection methods and techniques
- Data coding and cleaning
- Regulatory requirements for clinical data management
- Statistical analysis of clinical data
- Good Clinical Practice (GCP) guidelines
Why enroll for CliniLaunch Clinical Research Program
100% Placement Assistance
Industry-Relevant Training
Experienced Faculty
Benefits of a Clinical Data Management program
Level up your career! Unlock your future in clinical research with Clinical Data Management program that empowers you with the following benefits:

What roles await you in the market?
Be equipped with the skills and expertise to pursue exciting opportunities across the clinical research spectrum. Check out the various roles you can conquer with your CDM certification:
![]() Clinical Trial Manager (CTM): Lead the charge! Oversee all aspects of trials, managing budgets and timelines
Clinical Trial Manager (CTM): Lead the charge! Oversee all aspects of trials, managing budgets and timelines
![]() Clinical Data Manager (CDM): The data guru! Design data collection, manage databases, and ensure all is in compliance
Clinical Data Manager (CDM): The data guru! Design data collection, manage databases, and ensure all is in compliance
![]() Data Analyst/Biostatistician: Data leaders. Analyze data to identify trends and draw the right conclusions for the research
Data Analyst/Biostatistician: Data leaders. Analyze data to identify trends and draw the right conclusions for the research
![]() Clinical Data Associate (CDA): Data specialists who cleans, codes, and queries data for fine consistency
Clinical Data Associate (CDA): Data specialists who cleans, codes, and queries data for fine consistency
Why Clinical Data Management program from CliniLaunch?
Become a data management master
Gain skills that ensure data accuracy and compliance in clinical trials
Fast-track your career
Candidate’s chance to launch a rewarding career in Clinical Research with high-demand CDM skills
Become a regulatory expert
Understand and comply with essential regulations in CDM as per today’s norms
Master data analysis
Learn the in-trend industry-standard methods for data collection, cleaning, and analysis
Earn industry recognition
Gain a genuine certification to showcase your CDM expertise to your current and future employers
CliniLaunch advantage
Benefit from experienced faculty, real-world case studies, and dedicated placement assistance for your career goals
Topics And Skills Will be Covered In Clinical Research Program
Mastering niche skills for Clinical Data Management
- Clinical data management
- Statistical analysis
- Statistical analysis
- Data cleaning and coding
- Clinical trial operations
- Data analysis techniques
- Clinical trial documentation
Clinical Data Management Program syllabus
Curriculum Designed by Experts
A bachelor’s degree in life sciences, pharmacy, or a related field is preferred. Basic computer literacy and an interest in clinical research are also essential
Our comprehensive curriculum covers all aspects of Clinical Data Management (CDM), including:
- Clinical Data Management fundamentals
- Data collection methods and techniques
- Data coding and cleaning
- Regulatory requirements for clinical data management (GCP)
- Statistical analysis of clinical data
- Electronic Data Capture (EDC) systems
The duration of the program can vary depending on the chosen format (online or offline). We offer flexible options to suit your personal needs
The faculty for the Clinical Trial Management course is comprised of seasoned professionals with extensive experience in Clinical Trial Management. They bring in real-world insights and practical knowledge to the classroom
Absolutely, this is a certified course in India, where upon successful completion of the program, candidates receive a recognized certificate in Clinical Data Management. This credential will enhance your CV and demonstrate your expertise to potential employers
The Clinical Trial Management program offers a diverse range of career opportunities such as Clinical Data Coordinator, Clinical Data Associate, Clinical Data Manager, Data Analyst, and Regulatory Affairs Specialist.
Yes! We are dedicated to your career success. We provide comprehensive placement assistance services, that include aiding in your career growth and connecting you with top industry employers in the Clinical Research space
- Pharmaceuticals
- Biotechnology
- Medical Devices
- Contract Research Organizations (CROs)
- Hospitals and Research Institutions
For any further clarification about the program, curriculum, fees, and enrollment options, please contact us through our website or call us at +91 8904269998
FREE Career Counselling
We are happy to help you 24/7

Like the Program? Get started!

Program Certificate
To earn CliniLaunch’s post graduate diploma in clinical research certification, you must complete the designated coursework, assessments and any required practical projects or assignments. Once you complete the course work, you will receive the certificate.
Yes, CliniLaunch is certified and accredited by IAO (International Accreditation Organization), LSSSDC (Life Science Sector Skills Development Council), and NSDC (National Skill Development Council), the certificates are widely recognized and valued in the healthcare industry. The training programs at CliniLaunch are specifically designed to align with the best practices and ethical standards ensuring that the certificate holders are well-prepared for their chosen career path.
Holding a certificate from CliniLaunch validates your expertise in the field and enhances your credibility as a healthcare professional in the job market. The certification from CliniLaunch can open doors to job opportunities, career advancement, and higher earning potential in miscellaneous sectors in the healthcare industry.
Yes, CliniLaunch offers a range of certificate programs that can be completed online, providing flexibility and convenience for learners. Our online platform allows you to access course materials, lectures, and assignments from anywhere with an internet connection.
CliniLaunch, the best clinical research institute in India provides three programs under clinical research. We offer both short-term and comprehensive programs to suit different learning needs. The duration of PG Diploma in Clinical Research is 12 months, Advanced Diploma in Clinical Research is 6 months, and the duration for certification in clinical research is 3-4 months.
For financial assistance at CliniLaunch, we are committed to making education accessible to all individuals. We may offer financial assistance options such as scholarships, payments plans, or discounts for eligible students. Please contact our admission team for more information on available assistance programs.

Learn to detect, recognize, safeguard participant safety & trail integrity, develop & maintain protocol events, and respond to misconduct clinical trials.
Modal Box Title
- Courses
- Clinical Research
- Clinical Trial Management Course
Clinical Trail Management Course
Any Question? Happy to Answer
- 11k+ Students
Clinical trial management is the process of planning, organizing, and conducting clinical trials that evaluate the safety and efficacy of medical devices, new drugs, and procedures. CliniLaunch offers a comprehensive clinical trial management course to those early healthcare professionals and students who are interested in the field of clinical research. CliniLaunch’s experienced professionals have a proven track record of successfully training individuals with different aspects of clinical trials. They specifically focus on study design and protocol development to regulatory submissions and data management.
What is Clinical Trial Management?
Clinical trial management courses will help you develop cost-effective methods to treat diseases while increasing the quality of life. It also helps enhance successful new medical devices, biologics, and pharmaceutical launches by incorporating the genomics science for a personalized genetic prescription.
With the increasing opportunities in clinical trial management, the role of investigators is to support tasks revolving around regulatory compliance, scientific writing, marketing, data management, etc. In the field of clinical research, employment settings include medical device manufacturing companies, contract research organizations, hospitals, educational institutions, and independent contractors.
At CliniLaunch, clinical trial management course, training, and certification is offered online/in-person. The course curriculum offers an overview of the competencies necessary for a successful new product launch and development process.
What does clinical trial management cover?
- Introduction to Clinical Trials
- Good Clinical Practice (GCP)
- Clinical Trial Design and Development
- Clinical Trial Data Management
- Statistical Analysis
- Clinical Trial Monitoring & Auditing
- Clinical Trial Closeout & Reporting
Why enroll for CliniLaunch Clinical Trial Management course
100% Placement Assistance
Industry-Relevant Training
Experienced Faculty
Benefits of a Clinical Trial Management Course
Clinical trial courses are becoming the backbone of medical progress, ensuring new drugs and devices are safe and effective for usage in medical centers. Our Clinical Trial Management (CTM) program equips candidates with the knowledge and skills to excel in this critical field.

What roles await you in the market?
Offering a dynamic career path with opportunities for growth and leadership, Clinical Trial Management certified candidates have diverse career path options to pursue. Here’s a breakdown of key roles:
Clinical Trial Manager (CTM): A leader, overseeing all aspects of the trial. Managing budgets, timelines, and research teams.
Regulatory Affairs Specialist: Ensuring compliance with complex regulations set forth by bodies like CDSCO. Prepares and proposes regulatory documentation.
Medical Monitor (MM): A licensed physician overseeing all medical aspects of trials. Reviewer of participant eligibility and ensuring their safety.
Why Clinical Trial Management Course from CliniLaunch?
What is Clinical Trial Management?
Clinical trial management courses will help you develop cost-effective methods to treat diseases while increasing the quality of life. It also helps enhance successful new medical devices, biologics, and pharmaceutical launches by incorporating the genomics science for a personalized genetic prescription.
With the increasing opportunities in clinical trial management, the role of investigators is to support tasks revolving around regulatory compliance, scientific writing, marketing, data management, etc. In the field of clinical research, employment settings include medical device manufacturing companies, contract research organizations, hospitals, educational institutions, and independent contractors.
At CliniLaunch, clinical trial management course, training, and certification is offered online/in-person. The course curriculum offers an overview of the competencies necessary for a successful new product launch and development process.
What roles await you in the market?
Offering a dynamic career path with opportunities for growth and leadership, Clinical Trial Management certified candidates have diverse career path options to pursue. Here’s a breakdown of key roles:
![]() Clinical Trial Manager (CTM): A leader, overseeing all aspects of the trial. Managing budgets, timelines, and research teams.
Clinical Trial Manager (CTM): A leader, overseeing all aspects of the trial. Managing budgets, timelines, and research teams.
![]() Regulatory Affairs Specialist: Ensuring compliance with complex regulations set forth by bodies like CDSCO. Prepares and proposes regulatory documentation.
Regulatory Affairs Specialist: Ensuring compliance with complex regulations set forth by bodies like CDSCO. Prepares and proposes regulatory documentation.
![]() Medical Monitor (MM): A licensed physician overseeing all medical aspects of trials. Reviewer of participant eligibility and ensuring their safety.
Medical Monitor (MM): A licensed physician overseeing all medical aspects of trials. Reviewer of participant eligibility and ensuring their safety.
Gain comprehensive understanding
Master the intricacies of clinical trials, from study design to data analysis
Develop management skills
Equip yourself to manage all facets of a trial, ensuring smooth operation
Gain comprehensive understanding
Master the intricacies of clinical trials, from study design to data analysis
Master data analysis
Learn the in-trend industry-standard methods for data collection, cleaning, and analysis
Earn a recognized qualification
Boost your resume with a credential valued by employers
CliniLaunch advantage
Benefit from experienced faculty, real-world case studies, and dedicated placement assistance for your career goals.
Why Clinical Trial Management Course from CliniLaunch?
Gain comprehensive understanding
Master the intricacies of clinical trials, from study design to data analysis
Develop management skills
Equip yourself to manage all facets of a trial, ensuring smooth operation
Gain comprehensive understanding
Master the intricacies of clinical trials, from study design to data analysis
Master data analysis
Learn the in-trend industry-standard methods for data collection, cleaning, and analysis
Earn a recognized qualification
Boost your resume with a credential valued by employers
CliniLaunch advantage
Benefit from experienced faculty, real-world case studies, and dedicated placement assistance for your career goals
Topics And Skills Will be Covered In Clinical Research Program
Mastering niche skills for Clinical Data Management

- Clinical Trial Data Management

- Patient Recruitment & Retention Strategies
- Regulatory Compliance Expertise
- Clinical Trial Management Systems (CTMS)

- Statistical Analysis Fundamentals

- Risk management & GCP compliance
Topics And Skills Will be Covered In Clinical Research Program
Mastering niche skills for Clinical Trial Management
- Clinical Trial Data Management
- Patient Recruitment & Retention Strategies
- Regulatory Compliance Expertise
- Clinical Trial Management Systems (CTMS)
- Statistical Analysis Fundamentals
- Risk management & GCP compliance
Clinical Trial Management Course syllabus
Curriculum Designed by Experts
- DOWNLOAD CURRICULUM
What are the prerequisites for upskilling from Online Billing Courses?
To apply for an advanced diploma in medical coding online billing courses, you need to have at least a bachelor’s degree in life sciences and healthcare. However, with the degree, a basic understanding of medical coding terminology and computer literacy is required to become a medical coder.
How do I earn the Advanced Diploma in Medical Coding Certificate?
To earn the advanced diploma in medical coding course certificate, you must complete the designated coursework, assessments, and required practical projects. Once you complete the course successfully, you will be eligible to earn the certificate from CliniLaunch.
What is the curriculum for an Advanced Diploma in Medical Coding Course?
Module 1: Corporate Etiquette
Module 2: Non-Technical Training
(Aptitude + Communicatio)
Module 3: Introduction to Medical Coding
Module 4: Medical Terminologies
Module 5: Anatomy & Physiology
Module 6: Advanced Medical Coding Training
Module 7: CPC Certification Training
What are the learning materials included in the professional medical coder certification program?
The professional medical coder certification program includes comprehensive study material, including resources. You will be getting access to a learning management system to help you with study materials, online/live sessions and assignments.
What is the duration for advanced diploma in medical coding training program?
The duration of an advanced diploma in medical coding course is 6 months.
Which medical billing certification classes should I choose?
You can choose two medical billing certification classes which are advanced diplomas and certification in medical coding consists of 6 months and 2-3 months (depends on modules). CliniLaunch offers two modes: online/live sessions and offline/in-person classes.
What are the skills I will learn for the AAPC (American Academy of Professional Coders) examination?
Following are the skills you will be able to practically enhance for the American Academy of Professional Coders examination:
→ Medical Terminology
→ Anatomy and Physiology
→ ICD-10-CM and CPT coding Systems
→ Healthcare Regulations and Compliance
→ Project Work and Internship
→ Medical Record Review and Analysis
→ Software Proficiency
→ Coding Software Training
Who are the instructors?
The instructors are seasoned industry professionals with extensive learning and training experience in medical coding for your benefits.
What kind of job role should I consider after the completion of an advanced diploma in medical coding?
With the completion of Advanced Diploma in Medical Coding. You can unlock a spectrum of career opportunities and job roles within the healthcare sector.
Completing our Advanced Diploma in Medical Coding unlocks a spectrum of exciting career opportunities within the healthcare industry:
→ Medical Coder
→ Medical Biller
→ Coding Specialist
→ Healthcare Data Analyst
→ Clinical Documentation Specialist
Does CliniLaunch offer placement assistance for medical coding certification courses?
Yes, CliniLaunch offers placement assistance with “Placement Mentorship Program” and “Industry/Corporate Connect” to help you bridge the gap between academia and industry for medical coding certification courses.
Is placement assistance provided after the course completion or before?
CliniLaunch provides placement assistance during and after the course completion to help you connect with the corporate. T&C applied.
What are the sectors your medical coding certification can take us through?
CliniLaunch medical coding certification course can lead to a career in many sectors including:
→ Hospitals
→ Clinics
→ Insurance Agencies
→ Pharmaceuticals
→ Contract Research Organizations
→ Research Institutions
How can I know more about medical billing certification classes?
With an interest in medical billing certification classes online/offline, connect with CliniLaunch career counselors for the same.
Could you brief me about professional medical coder certification?
Professional medical coder certification is a credential for healthcare coding and billing enthusiasts. A certified professional coder certification with an advanced diploma in medical coding course shows you have learned a certain medical coding system with the basics. It will help you understand how to translate medical data of patients into codes. If you choose to earn it, you will have ample options in the healthcare industry.
FREE Career Counselling
We are happy to help you 24/7
A bachelor’s degree in any discipline is preferred. And, basic knowledge of biology and healthcare is beneficial for a better understanding.
The duration of the program can vary depending on the chosen format (online or offline). We offer flexible options to suit your personal needs.
The faculty for the Clinical Trial Management course is seasoned professionals with extensive experience in Clinical Trial Management. They bring in real-world insights and practical knowledge to the classroom.
This is a certified course in India, where upon successful completion of the program, candidates receive a recognized Clinical Trial Management Certification. This credential will enhance your CV and demonstrate your expertise to potential employers.
The Clinical Trial Management program offers a diverse range of career opportunities. You can pursue roles like Clinical Research Associate (CRA), Clinical Trial Manager (CTM), Data Manager, Regulatory Affairs Specialist, and many more.
Yes! We are dedicated to your career success. We provide comprehensive placement assistance services that include aiding in your career growth and connecting you with top industry employers in the Clinical Research space.
We request candidates to explore the detailed program information on our website or contact our admissions team directly before choosing the course. Our team can help you clarify all specific questions candidates may have and guide you through the application process.
FREE Career Counselling
We are happy to help you 24/7
Like the Program? Get started!

Clinical Trial Management Course details
Yes! Risk management and GCP (Good Clinical Practices) also available in the clinical trial classes. CliniLaunch provides training and placement assistance to students with strict adherence to GCP guidelines ensuring ethical conduct and data integrity.
Yes! The clinical trial data management program equips students with a solid foundation of statistical analysis that comes under clinical trial courses.
CliniLaunch keeps updating the curriculum for pharmacovigilance in clinical trials and other courses on a quarterly basis. It will help trainers to make students aware of the latest trends, teaching methods and techniques while helping them
The program keeps pace with the latest developments happening in clinical trial regulations time-to-time. You’ll gain a comprehensive understanding of relevant guidelines set forth by bodies like CDSCO, India, and develop the skills to navigate the regulatory approval process effectively
- Contract Research Organizations (CROs)
- Pharmaceutical & biotechnology companies
- Medical Device Companies
- Hospitals & Research Institutions
A: For any further clarification about the program, curriculum, fees, and enrollment options, please contact us through our website or call us at + 91-8904269998
Program Certificate
To earn a clinical trial management certification in clinical research, you must complete the designated coursework.
Yes, CliniLaunch is certified and accredited by IAO, NSDC, EBVTR, IAF, etc. Therefore, clinical trial management certification is recognized by the industry and its peers.
Holding a certificate from CliniLaunch validates your expertise in the field and enhances your credibility as a healthcare professional in the job market. Clinical Trial Certificate Program can open doors to job opportunities, career advancement, and higher earning potential in miscellaneous sector in healthcare industry.
Yes, you can pursue clinical trials classes online based on your convenience. CliniLaunch’s LMS (Learning Management System) allows you to access courses, materials, lectures, and assignments from anywhere with an internet connection.
Clinical trial management certification program comes under Advanced and PG Diploma in Clinical Research. It depends on the program you choose to earn a certificate from CliniLaunch.
Yes! Financial assistance is available for the clinical trial certificate program. Consider connecting with your career counselor for the same.

Adhere to regulatory compliance, commit to follow the guidelines, and conduct clinical trials for pharmaceutical, medical devices, understand its approval processes.
Modal Box Title
- Courses
- Clinical Research
- Regulatory Affairs Course
Regulatory Affairs Course
Any Question? Happy to Answer
- 11k+ Students
Regulatory Affairs is the field that is focused on ensuring the safety, effectiveness, and quality of drugs and medical devices by navigating the complex web of regulations set as per the standards by government agencies. This upskilling program enables an already existing regulatory affairs personnel in the pharmaceutical regulatory industry to understand all current diagnostic and medical device regulations and further develop the necessary skills to work successfully in the dynamic world of regulatory affairs. The main goal, devise and implement global strategies for drug, biologic, and device development and evaluation. Apply learnings of basic and applied pharmaceutical sciences further in drug and biologics discovery and development. The objective for candidates is to master and expand their job responsibilities and opportunities in new areas after completing the regulatory affairs course. CliniLaunch’s Regulatory Affairs is India’a finest upskilling program that equips candidates with the knowledge and expertise to excel in this dynamic field due to the thorough and intensive curriculum delivered by the renowned faculties
Why enroll for the CliniLaunch Regulatory Affairs course?
Industry-driven curriculum
Curriculum focused on practical application
Job-oriented curriculum
Master eCTD submissions skills
Industry expert-led instruction
Career advancement support
Benefits of a Regulatory Affairs Upskilling course
Unlock the growth potential of a regulatory affairs upskilling program by gaining the expertise to thrive in the ever-evolving world of drug regulations in the pharmaceutical industry.

What roles await you in the market?
Shape your future in Regulatory Affairs by exploring a diverse range of roles necessary for obtaining new drugs and devices to the market safely and effectively. Here’s a glimpse into a few of the rewarding career paths candidates can pursue:
![]() Clinical Data Coordinator/Associate: Ensures accurate data collection, manages CRFs (Case Report Forms), and performs initial data cleaning
Clinical Data Coordinator/Associate: Ensures accurate data collection, manages CRFs (Case Report Forms), and performs initial data cleaning
![]() Clinical Data Manager: Oversees data management processes, ensures regulatory compliance, and collaborates with statisticians for analysis
Clinical Data Manager: Oversees data management processes, ensures regulatory compliance, and collaborates with statisticians for analysis
![]() Data Entry Specialist :Accurately enters clinical trial data into electronic databases and performs routine data validation checks
Data Entry Specialist :Accurately enters clinical trial data into electronic databases and performs routine data validation checks
![]() Clinical Data Analyst: Analyzes and interprets clinical trial data, prepares data summaries, and designs projections for regulatory reports
Clinical Data Analyst: Analyzes and interprets clinical trial data, prepares data summaries, and designs projections for regulatory reports
Why choose the Regulatory Affairs course from CliniLaunch?
Benefits of a Regulatory Affairs Upskilling course
Drug regulatory affairs courses play a crucial role in ensuring accessibility to safe and effective medications for patients. Some of the benefits of regulatory affairs in clinical research offer diverse career opportunities, competitive salary and others.
What roles await you in the market?
Let’s shape your future with CliniLaunch regulatory affairs training by exploring a diverse range of roles. These roles are necessary to obtain new drugs and devices in the market safely and effectively. Here is a glimpse into a few rewarding career paths you can pursue.
Clinical Data Associate/Coordinator: Ensure accuracy in data collection, manage case report forms, and perform initial data cleaning.
Clinical Data Manager: Oversee data management processes, ensure regulatory compliance, and collaborate with statisticians for analysis.
Data Entry Specialist: Accurately enters clinical trial data into electronic databases and performs routine data validation checks.
Clinical Data Analyst: Analyze and interpret clinical trial data, prepare data summaries, and design projections for regulatory reports.
Clinical Coding Specialist: Standardize medical code based on conditions, procedures, and ADEs (Adverse Drug Events) for data consistency.
Uniqueness factor
One-of-its-kind upskilling program for regulatory affairs in clinical research
Enhanced expertise
Navigate the intricacies of drug regulations and ensure compliance for your organization
Industry-expertise from the very best
Experienced faculty providing candidates with the right hands-on training
Increased earning potential
Professionals of regulatory affairs in pharma are in high demand and upskilling command a competitive pay package
Advance your career
Open doors to an exciting career of regulatory affairs in the clinical research and pharma sector.
Boost your resume
Stand out in the market with employers for in-demand pharmacovigilance in clinical research.
Why choose the Regulatory Affairs course from CliniLaunch?
Uniqueness factor
One-of-its-kind upskilling program for pharmacy students in India
Enhanced expertise
Navigate the intricacies of drug regulations and ensure compliance for your organization
Industry-expertise from the very best
Experienced faculty providing candidates with the right hands-on training
Increased earning potential
Regulatory Affairs professionals are in high demand, which shows that this program can equip candidates with the skills to command a competitive pay package
Advance your career
Open doors to exciting career clinical research opportunities in Pharma, CROs, and regulatory affairs
Boost your resume
Be a market standout with employers for in-demand Pharmacovigilance skills
What topics are covered in the Pharmacovigilance course cover?
Mastering niche skills for Medical Writing course
- Indian pharma regulations
- Practical regulatory skills
- eCTD expertise
- Global regulatory knowledge
- Industry-ready skills
- Regulatory writing skills
- Compliance expertise
What topics are covered in the Pharmacovigilance course cover?
Mastering niche skills for Medical Writing course

- Compliance expertise
- Regulatory writing skills

- Industry-ready skills

- Global regulatory knowledge

- eCTD expertise

- Practical regulatory skills

- Indian pharma regulations
Regulatory Affairs syllabus Course syllabus
Curriculum Designed by Experts
- DOWNLOAD CURRICULUM
Regulatory Affairs course FAQs
The regulatory affairs training is designed specifically for healthcare professionals, clinical researchers and anyone interested in pharmacovigilance.
A bachelor’s degree in life sciences, pharmacy, or a related field is preferred. Basic computer literacy and an interest in regulatory affairs in clinical research.
- Essential document types
- ICH-GCP guidelines
- Regulatory expertise in mastering ICH-GCP guidelines
- Regulatory documents
- Regulatory writing skills
- How to ensure data and communications meet global regulatory standards
- Scientific writing skills to transform complex data into clear and impactful communication
- Gain project management expertise
After completion of the regulatory affairs course, you will be qualified for the following different roles.
→ Clinical Data Associate/Coordinator
→ Clinical Data Manager
→ Data Entry Specialist
→ Clinical Data Analyst
→ Clinical Coding Specialist
Additionally, it can even lead to Clinical Trial Assistant (Regulatory Affairs focused) and Medical Writer (Regulatory Affairs Focused) too.
To excel in regulatory affairs in pharma industry, you need to execute your problem solving skills. It will help you navigate the issues creatively and efficiently to minimize the impact on the regulatory process and business operations.
Yes! CliniLaunch offers career guidance during regulatory affairs training to help you find the perfect job opportunities in the healthcare sector.
The duration of the program may vary depending on the modules you chose under the course certification in clinical research, advanced diploma in clinical research, or postgraduate diploma in clinical research.
Absolutely yes! The regulatory affairs course comes under advanced and PG Diploma in Clinical research courses. You need to consider clinical research sub-courses to get the certification from CliniLaunch.
Yes! CliniLaunch offers placement assistance for regulatory affairs in pharma for your career success. The placement assistance consists of “Placement Mentorship Program” and Corporate/Industry Connects.
- Pharmaceutical Industry
- Biotechnology Industry
- Medical Device Industry
- Food and Beverage Industry
- Cosmetics and Dietary Supplement Industry
- Contract Research Organizations (CROs)
- Government Agencies
- Consulting Firms
FREE Career Counselling
We are happy to help you 24/7
Like the Program? Get started!

Program Certificate
To earn a certificate for regulatory affairs in clinical research, you need to enroll or register for Clinical Research programs – either advanced diploma or postgraduate diploma.
Yes! CliniLaunch is accredited and recognized by IAO, NSDC, IAF, EBVTR and is under the membership of LSSSDC. Therefore, the certifications from CliniLaunch are recognized worldwide.
Holding a certification for a regulatory affairs course is to get the validation from industry experts. This will enhance your credibility in the healthcare settings and help you gain access to the job market nationally and internationally.
Yes, CliniLaunch offer online/live training sessions for you. With the access to Learning Management System, you will be able to pursue regulatory affairs training online with course material, live training sessions, and assignments.
Yes! During the admission process, you will get financial assistance from the financial team or your career counselors.
Get certified with CliniLaunch’s pharmacovigilance course with a comprehensive curriculum driven by industry experts to enhance your career prospects.
Modal Box Title
- Courses
- Clinical Research
- Pharmacovigilance Course
Pharmacovigilance Course
Any Question? Happy to Answer
Pharmacovigilance in Clinical Research, Career in Pharmacovigilance, Pharmacovigilance Training Online, Pharmacovigilance Training Course
CliniLaunch bridge the gap between academia and industry - Pursue a career in pharmacovigilance.
The pharmacovigilance course is a sub-course of clinical research consisting of the science and activities involved in assessing The pharmacovigilance course is a sub-course of clinical research consisting of the activities and science involved in assessing, detecting, understanding, and preventing advance events or any drug-related problems. The pharmacovigilance in clinical research helps medical professionals to monitor safety of pharmaceutical products and ensure public health.
CliniLaunch’s meticulously designed module takes a close look at the important roles pharmacovigilance plays throughout clinical research and development, intersecting with all other departments. This pharmacovigilance course will help you gather and evaluate ADR reports, keep track of medicine to identify trending patterns, determine actions to improve medicine safety, and provide information to users efficiently and safely.
What is Pharmacovigilance?
Pharmacovigilance is the vital science of monitoring the safety of medications after they are introduced in the market. Upskill for a future in Pharmacovigilance – Propel your career with CliniLaunch Research Institute’s comprehensive upskilling program. This industry-aligned course equips candidates with the right knowledge and expertise to excel in identifying, assessing, and preventing adverse drug reactions (ADRs) that are based on the DIA Safety and Pharmacovigilance competency framework developed with industry experts working in the field.
Whether you’re a healthcare professional seeking to expand your skillset or a newcomer aiming to launch a fulfilling career in Pharmacovigilance, CLRI’s upskilling program empowers candidates to become a valuable asset in safeguarding patient well-being.
With the latest industry practices and regulatory guidelines navigating the evolving field of Pharmacovigilance, get ready to upskill for a career in medication safety.
Learnings Pharmacovigilance Course offers
- Master basics of Pharmacovigilance
- Gather expertise in Pharmacovigilance fundamentals and impact on the clinical research
- Gain experience in differentiating adverse events vs. side effects vs. drug reactions
- Pharmacovigilance software and database know-how
- Regulatory compliance and guidelines as per the clinical norms
- Understand the impact on medication safety, enhancing patient safety, and public health
- Industry best practices. Distinguish how to handle adverse events, side effects, and drug reactions in clinical research
- Understand case processing and its impact on patient safety
- Stand out in the job market sector
Pharmacovigilance Training Course offers:
Pharmacovigilance Introduction
Regulatory Knowledge
ADR Reporting
Pharmacovigilance Project Management
Risk minimization Methods
Safety Monitoring
Benefits of a medical writing course
Join the medical writing program from CliniLaunch Research Institute in clinical research equipping candidates with all the skills to create clear, accurate, and impactful documents that drive successful clinical trials.

What roles await you in the market?
Medical writing skills equip candidates for a diverse range of rewarding yet exciting careers. Here’s a glimpse into a few of the rewarding career paths candidates can pursue:
![]() Medical Safety Officer (MSO): Overseeing the entire pharmacovigilance course, developing risk management plans, and providing medical expertise
Medical Safety Officer (MSO): Overseeing the entire pharmacovigilance course, developing risk management plans, and providing medical expertise
![]() Regulatory Affairs Specialist (Pharmacovigilance): Prepares and submits pharmacovigilance reports, and cooperates with regulatory bodies for compliance
Regulatory Affairs Specialist (Pharmacovigilance): Prepares and submits pharmacovigilance reports, and cooperates with regulatory bodies for compliance
![]() Pharmacovigilance Auditor: Conducting audits to ensure compliance with pharmacovigilance regulations and best practices in the industry
Pharmacovigilance Auditor: Conducting audits to ensure compliance with pharmacovigilance regulations and best practices in the industry
![]() Pharmacovigilance Data Analyst: Analyzing data to identify trends and potential safety risks associated with medications
Pharmacovigilance Data Analyst: Analyzing data to identify trends and potential safety risks associated with medications
Pharmacovigilance Training Online
Pharmacovigilance is the vital science of monitoring the safety of medications after they are introduced in the market. Upskill for your future career with pharmacovigilance training online and propel your career moving forward. This industry-aligned course equips you with the right knowledge and expertise to excel in the field of clinical research. In general, pharmacovigilance professionals identify, assess, and prevent adverse drug reactions (ADRs) based on drug information Association (DIA) safety and pharmacovigilance.

- Collect and manage data for your clinical research studies and documentation and learn how to build and capture electronic data instruments.

- Learn to detect, recognize, safeguard participant safety & trail integrity, develop & maintain protocol events, and respond to misconduct in clinical trials.

- Be willing to create clinical documents, scientific papers, regulatory documents, and patient information leaflets with CliniLaunch’s medical writing course.

- Get certified with CliniLaunch’s pharmacovigilance course with a comprehensive curriculum driven by industry experts to enhance your career prospects.

- Adhere to regulatory compliance, commit to follow the guidelines and conduct clinical trials for pharmaceutical and medical devices.
Enhance key skills with Pharmacovigilance in clinical research
Clinical Data Analysis
Clinical Data Management
Medical and Scientific Concepts
Coordination with Team
Writing and Editing
Effective Communication
Pharmacovigilance Course with Placement
Pharmacovigilance course with placement assistance will help you enhance your knowledge to serve in the healthcare industry. Without proper academic knowledge and coverage in the pharmacy graduation or post-graduation, it may lead to lack of proper knowledge to serve the healthcare industry. CliniLaunch Research Institute offers complete training for pharmacovigilance, including regulatory compliances, adverse drug events (ADEs), adverse drug reactions (ADRs), risk management, and signal detection.
Mastering niche skills for Pharmacovigilance course
- Drug safety monitoring and data analysis
- Regulatory compliance savvy
- Case processing efficiency
- Pharmacovigilance software expertise
- Industry best practices for Pharmacovigilance
- Advanced Pharmacovigilance practices
Why Pharmacovigilance Training Course?
Industry-led Curriculum
→ Industry Standards
→ In-depth Analysis
Experienced Instructors
→ Industry Expert Trainers
→ Dedicated Mentors
Hands-on Training
→ Live Training Sessions
→ Industry Case Studies
Accreditations/Membership
→ IAO, BRIT QUALIS, NSDC
→ LSSSDC
Career Support Services
→ Placement Mentorship Program
→ Corporate Connect
Take a first step towards your bright future!
Start your journey today!
Common Curriculum for Pharmacovigilance Training Course – Download Now
- Medical Writing in Pharmacovigilance
- Drug Safety & Data Management
- Risk Management & Minimization Plans
- Pharmacoepidemiology
- Adverse Drug Reaction Reporting
- Regulatory Requirements
- Pharmacovigilance in Clinical Research
- Introduction to Pharmacovigilance
FREE Career Counselling
we are happy to help you 24/7
Pharmacovigilance syllabus course syllabus
Curriculum Designed by Experts
Pharmacovigilance Course – FAQs
Pharmacovigilance is the science and practice to detect, assess, understand, and prevent adverse effect of drugs. It is a crucial aspect of ensuring patient safety and maintaining the integrity of the pharmaceutical sector.
Pharmacovigilance professionals play a crucial role in protecting public health by preventing ADEs and improving drug safety. A pharmacovigilance professional is responsible for monitoring and evaluation of the drug safety after they have been approved for use. Pharmacovigilance professionals collect, analyze, and interpret data on ADRs to identify potential safety risks. This may involve case reports, conducting safety assessments, and collaborating with healthcare professionals, regulatory agencies, and pharmaceutical companies.
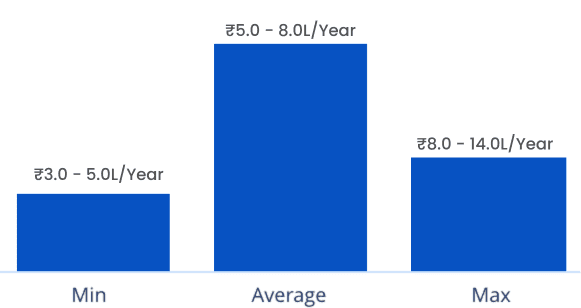
The highest salaries for pharmacovigilance professionals are typically earned by those who earned a postgraduate diploma in clinical research with extensive experience in the field. However, it also depends on the experience, and their highest qualification. Here are some salary range or expectations:
- Entry-level: ₹3-4 Lakhs per annum
- Mid-level: ₹6-10 Lakhs per annum
- Senior-level: ₹10-15 Lakhs per annum
- Medical Safety Specialists: ₹20 Lakhs or more per annum
Pharmacovigilance professionals monitor the safety of pharmaceutical products and ensure public health. They detect, assess, understand, and prevent adverse effects or other drug-related problems.
To identify signals of medicine safety i.e. unknown or poorly characterized adverse events in relation to a medicine or a combination of medicines and/or its use. To undertake assessment of risk and options for risk management.
CliniLaunch is the best institute for pharmacovigilance courses.
To start your career in pharmacovigilance, you need at least a bachelor’s degree which is specifically required in the field of life sciences, pharmaceutical or health sector.
To get a pharmacovigilance job, you must develop your practical skills along with communication and negotiation skills. In collaboration with CliniLaunch corporate partners, it will be easier for you to find the best suitable company for your career in pharmacovigilance.
CliniLaunch is the best institute for pharmacovigilance training courses. CliniLaunch holds education and training standards with accreditations and certifications from IAO, NSDC, IAF, Brit Qualis UK Ltd., and EBVTR.
Clinical Research course from CliniLaunch is best for pharmacovigilance training online in India. CliniLaunch offers 100% placement assistance, career fair and networking opportunities for you.
Pharmacovigilance is a promising and rewarding career choice for those professionals interested in the healthcare industry.

Be willing to create clinical documents, scientific papers, regulatory documents, and patient information leaflets with CliniLaunch’s medical writing course to provide comprehensive insights.
Modal Box Title
- Courses
- Clinical Research Training in Hyderabad
Medical Writing
Any Question? Happy to Answer
Medial Writing
- 11k+ Students
Looking for the best clinical research institute in Chennai to advance your career in healthcare? Look no further! CliniLaunch helps you with your career development and placement at the right place. Enroll today and you will be able to elevate yourself with the skills in disease prognosis and advance your methods to approach treatment or diagnoses.
Chennai is the city known for “healthcare innovation hub” with CROs (Contract Research Organizations) for successful clinical trials, and clinical research institute. As the home of healthcare and innovation, Chennai is the center to advance your healthcare career. This is where CliniLaunch will help you boost your knowledge and skills with high-quality industry expert training. Learn clinical trials, data management, pharmacovigilance, regulatory affairs, and more.
Medical Writing Course offers Skills:
- Medical Writing Fundamentals
- Write Compelling Documentations
- Sharpen Scientific Research
- Master Scientific Writing
- Leverage Scientific Data
- Manuscript Creation for Publication
Medical Writing Training Online
Boost your medical writing training online or offline and gain exposure to healthcare industry applications. With top-tier medical writing expert trainers, CliniLaunch will help you advance your career in the clinical research domain. As medical writing is a sub-course of clinical research, there are 4 more courses that you should consider with this training.

- Data Insight
- Analytical Excellence
- Outcome Enhancement
Medical Writing Course with Placement
Medical writing course with placement assistance at CliniLaunch will enhance your medical and scientific writing skills. Learn effective medical writing techniques and help you implement effective strategies for creating and engaging and accurate clinical documents. It is an exciting career choice and you should pursue medical writing in clinical research for the right reasons. CliniLaunch will help you lead and excel in the field of medical writing with its placement mentorship program.
Why choose the medical writer Certification course from CliniLaunch?
Become a subject matter expert
Acquire in-depth knowledge of medical writing in clinical research, medical terminology and regulatory affairs.
Regulatory knowledge
Attaining to the ICH-GCP guidelines we ensure a candidate’s writing skills meet global regulatory standards
High-demand field
The clinical research industry is booming, creating a strong job market for medical writers
Contribute to healthcare advancement
By ensuring clear communication, one plays a vital role in bringing new treatments to patients
Mastering the right communication skills
Mastering the art of translating complex scientific data into clear and impactful medical writings for diverse audiences
Diverse career options
Work with pharmaceutical companies, and CROs for a variety of clinical research projects and betterment of their career
Why Medical Writing Training Online?
Industry-led Curriculum
→ Industry Standards
→ In-depth Analysis
Experienced Instructors
→ Industry Expert Trainers
→ Dedicated Mentors
Hands-on Training
→ Live Training Sessions
→ Industry Case Studies
Accreditations
→ IAO, BRIT QUALIS, NSDC
→ LSSSDC
Career Support Services
→ Placement Mentorship Program
→ Corporate Connect
Take a first step towards your bright future!
Start your journey today!
Curriculum Designed by Experts
Common Curriculum for Medical Writing in Clinical Research
- Introduction to Medical Writing
- Medical Writing in Clinical Research
- Scientific Writing
- Drug Development Process-An Overview
- Pre-Clinical and non-Clinical Writing
- Medical Writing for Clinical Trials
- The Post-Marketing Phase
FREE Career Counselling
We are happy to help you 24/7
Like the Program? Get started!

Clinical Research Institute in Chennai - FAQs
Medical writing is the creation of well-structured scientific documents that include clinical research documents, content for healthcare websites, health magazines, journals, and news.
A medical writer involves themselves in different types of scientific documents including regulatory and research-related documents, disease or drug-related educational and promotional literature, publication articles like journal manuscripts and abstracts, healthcare website content, and health related magazine or news.
To qualify for medical writing, you may require a background in life sciences, medical sciences, or a pharmacy degree. It may require a strong foundation in science to help you understand the medical content that you will be writing about. Moreover, some medical writing professionals choose to further their education by pursuing advanced degrees or certificates in medical writing.
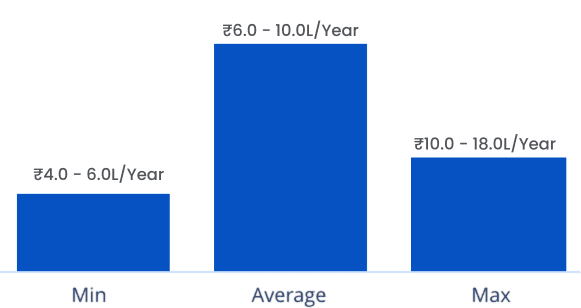
According to Ambitionbox, medical writer salaries in India may range between ₹2.0 Lakhs to ₹10.0 Lakhs with an average annual salary of ₹5.7 Lakhs.
Medical writing aims at healthcare professionals (HCPs), whereas scientific writing specifically aims at individuals with a professional or personal interest based on broader scientific topics. Each content creator should be a subject matter expert in their core areas.
A medical transcriptionist should be well-versed with correct spelling of all terms and words with an understanding of correcting medical terminology. Whereas, a medical writer needs to have a wide variety of skills to write on different topics based on the various target groups.
Microsoft Word or Google Docs are the best softwares used for medical writing in the world. They are commonly used to write and format documents such as clinical study reports, scientific research article journals, and regulatory submissions.
Most professional publications adhere to the American Medical Association (AMA) or Council of Science Editors (CSE). These style guides may be updated constantly and writers remain current with the latest style guides with its requirements.
MLA writing style is specifically used for humanities which is typically for literature research. If you are writing journals for medicine and sciences, follow Vancouver Style. The National Library of Medicine maintains Vancouver and MLA writing styles.
Manuscript in medical writing is an art to present complex scientific data into manuscript format. To create a good one, the medical writer should have a flair of writing in different styles, formats, and size of writing expertise in areas such as therapeutic area of expertise, clinical trials, drug development process and project management skills.
You can enroll at CliniLaunch for PG diploma in clinical research with placement assistance under which medical writing course comes in.
Achievements of Our Learners
9.5%
Avg. Salary Hike
100+
Hiring Partners
5/6
Experienced Career Growth
200+
Career Transitions
Our Hiring Partners








Hear From Our Students
Sounds Good? What you hear from our Students?
Begin your journey now!
Benefits of Learning from CliniLaunch Research Institute
CliniLaunch Research Institute
Non - Accredited Institute
Accredited Institution
CliniLaunch is an accredited institution, enhancing the credibility of the certificate.
Global recognition ensures better career prospects and industry acceptance.
Lack of accreditation may limit recognition of the certificate in the industry.
Employers may question the credibility of the course from non-accredited institutes.
Expert Faculty
Learn from experienced industry professionals and renowned faculty.
Access to high-quality teaching materials and personalized guidance.
Courses from other institutes may lack experienced industry professionals as faculty.
Quality of teaching may be compromised, affecting the learning experience.
Diverse Career Opportunities
Graduates gain access to a wide range of career options in clinical research and healthcare sectors.
Comprehensive placement assistance ensures better job prospects and career growth.
Graduates may face challenges in accessing a diverse range of career opportunities.
Placement assistance may not be provided, making it harder to secure job placements.
Flexible Learning Options
CliniLaunch offers flexible learning modes, including online and offline options.
Students can choose learning schedules that suit their needs and commitments.
Other courses may offer rigid learning structures with limited flexibility in learning modes.
Limited access to online resources and interactive sessions may hinder the learning process.
Networking and Support
Students benefit from networking opportunities with industry experts and fellow professionals.
Access to exclusive events and a supportive community enhances learning experience and career advancement.
Students may miss out on networking opportunities with industry professionals.
Limited access to exclusive events and networking platforms may hinder career growth.

Clinical Data Management Course
Collect and manage data for your clinical research studies and documentation and learn how to build and capture electronic data instruments.

Clinical Trial Management Course
Learn to detect, recognize, safeguard participant safety & trail integrity, develop & maintain protocol events, and respond to misconduct clinical trials.

Regulatory Affairs Course
Adhere to regulatory compliance, commit to follow the guidelines, and conduct clinical trials for pharmaceutical, medical devices, understand its requirements and approval processes.

Pharmacovigilance Course
Get certified with CliniLaunch’s pharmacovigilance course with a comprehensive curriculum driven by industry experts to enhance your career prospects.

Medical Writing Course
Be willing to create clinical documents, scientific papers, regulatory documents, and patient information leaflets with CliniLaunch’s medical writing course to provide comprehensive insights.
What Sets CliniLaunch Apart

Placement Assistance
Strong network of recruiters from the healthcare, pharmaceutical, and biotechnology industries and offer placement assistance to students.
Industry Expert Trainers
Equip yourself with skills and knowledge under the mentorship of experienced faculties with over 17 years of experience in the field of healthcare research and training.
Learning Management System
Embark on a transformative learning experience with our state-of-the-art Learning Management System!
Non-Technical and Technical Sessions
Go beyond the textbook with a well-rounded foundation balancing essential technical and non-technical skills needed to thrive in healthcare, IT and Pharma.
Job Oriented Programs
Get comprehensive job-oriented programs to empower you with the skills and knowledge you need to succeed in the dynamic and competitive healthcare sector.
What Sets CliniLaunch Apart

Placement Assistance
Strong network of recruiters from the healthcare, pharmaceutical, and biotechnology industries and offer placement assistance to students.
Industry Expert Trainers
Equip yourself with skills and knowledge under the mentorship of experienced faculties with over 17 years of experience in the field of healthcare research and training.
Learning Management System
Embark on a transformative learning experience with our state-of-the-art Learning Management System!
Non-Technical and Technical Sessions
Go beyond the textbook with a well-rounded foundation balancing essential technical and non-technical skills needed to thrive in healthcare, IT and Pharma.
Job Oriented Programs
Get comprehensive job-oriented programs to empower you with the skills and knowledge you need to succeed in the dynamic and competitive healthcare sector.
A Student’s Journey



Equip yourself with skills and knowledge required to be successful in the healthcare-pharma or healthcare-IT industry. Enhance your communication and personality.
Earn credentials through online or in-person programs validating the enhancement of your skills and expertise in the healthcare-IT and pharma sector.
Gain access to volunteer, internship, and placement opportunities and apply real-world applications in healthcare settings like hospitals, CROs, and pharma companies.
Industry-Ready Training
Equip yourself with skills and knowledge required to be successful in the healthcare-pharma or healthcare-IT industry. Enhance your communication and personality.
Certified Courses
Earn credentials through online or in-person programs validating the enhancement of your skills and expertise in the healthcare-IT and pharma sector.
Get Placed
Gain access to volunteer, internship, and placement opportunities and apply real-world applications in healthcare settings like hospitals, CROs, and pharma companies.
Level up your medical career with CliniLaunch.
Real people. Real results.
Master in-demand skills and knowledge
Propel your clinical research career with CliniLaunch

Unlock your potential Mentorship advantage
Personalized industry-experts with career advice and support to your choices.
Navigate your medical career with confidence
Unlock your potential with upskilling in what you truly enjoy

CliniLaunch recent placed students


















Testimonials
Upskilling does make a difference. Graduates speak out. Hear what our students and professionals are saying about their upskilling journey with CliniLaunch.
FAQs for Clinical Research Sub-Courses in Bangalore
Medical writing is the creation of well-structured scientific documents that may include clinical research documents, content for healthcare websites, health magazines, journals, and news.
A medical writer can work on different documentations. They research, write and refine different scientific documents including regulatory and research related documents, disease or drug educational and promotional literature, publication journal articles such as manuscripts, abstracts, synopsis, healthcare website content, magazines, or news.
The qualification for medical writing in clinical research includes a background in life science, medical sciences or a degree in pharma. It requires a strong foundation in science to help you understand the medical content in a clear and concise manner. Furthermore, some healthcare professionals choose to further their education by pursuing advanced degrees or certificates in medical writing.
Based on the suggestions from Ambitionbox, salaries of medical writers in India may range between ₹2.0 to ₹10.0 Lakhs.
Medical Writing specifically aims at healthcare professionals based on broader scientific topics. Each content creator should be a subject matter expert in their core areas.
A medical transcriptionist should be well-versed with correct spelling of all terms and words with an understanding of correcting medical terminology. Whereas, a medical writer needs to have a wide variety of skills to write on different topics based on the various target groups.
Microsoft Word or Google Docs are the best softwares used for medical writing in the world. They are commonly used to write and format documents such as clinical study reports, scientific research article journals, and regulatory submissions.
MLA writing style is specifically used for humanities which is typically for literature research. If you are writing journals for medicine and sciences, follow Vancouver Style. The National Library of Medicine maintains Vancouver and MLA writing styles.
Manuscript in medical writing is an art to present complex scientific data into manuscript format. To create a good one, the medical writer should have a flair of writing in different styles, formats, and size of writing expertise in areas such as therapeutic area of expertise, clinical trials, drug development process and project management skills.
You can enroll at CliniLaunch for PG Diploma in clinical research with placement assistance under which medical writing course comes in.
This program is specifically designed for healthcare professionals who are interested in clinical research including pharmacovigilance, regulatory affairs, clinical trial management, medical writing, and clinical data management.
A bachelor’s degree in life sciences, pharmacy, or a related field is preferred. Note: Basic computer literacy and an interest in clinical research is also essential.
- Essential document types
- ICH-GCP guidelines
- Regulatory expertise in mastering ICH-GCP guidelines
- Regulatory documents
- Regulatory writing skills
This program can help candidates qualify for roles like Clinical Data Associate/Coordinator, Clinical Data Manager, Data Entry Specialist, Clinical Data Analyst, Clinical Coding Specialist, and many more.
You will gain experience in industry-standard software like MS word, reference management tools (e.g. EndNote, FootNote), and potentially specialized clinical trial software.
Yes, CliniLaunch offers career guidance to help candidates find the perfect job opportunity in regulatory affairs.
Regulatory Affairs course is a module included in Clinical Research Program, the duration of the course may vary. Please connect with your career counselor for the same.
Absolutely, whether you enroll for modules or a full clinical research course, you will be getting a certificate after the completion of the program.
Yes! CliniLaunch offers placement assistance with “Placement Mentorship Program” while providing networking opportunities for the same after completion of pharmacovigilance course under PG Diploma in Clinical Research.
Yes, the program incorporates case studies, simulations, and practical assignments to enhance the candidate’s learning.
Yes, you can gain access to the course materials till post completion of the course for 1 month.
Yes, the pharmacovigilance course covers commonly used pharmacovigilance software like Argus/ARIS G which is mostly used in the healthcare industry.
To earn a certificate for pharmacovigilance in clinical trials, you need to complete the designated coursework, assessments and any required practical projects or assignments.
For a clinical trial management course, you need to consider the eligibility criteria given for the clinical research course. You must be from a life science, or medical science background.
The duration of the program may vary. Please connect with your career counselor for the same.
The clinical trial management program offers a diverse range of career opportunities. You can pursue roles such as Clinical Research Associate (CRA), Clinical Trial Manager (CTM), Pharmaceutical Regulatory Affairs Specialist, Clinical Data Manager, etc.
Explore, clinical trial management course or contact your career counselor for the same before choosing the course. They will take you through the overall application process.
CliniLaunch’s comprehensive curriculum covers all aspects of Clinical Data Management training includes:
→ Clinical Data Management Fundamentals
→ Data Collection Methods and Techniques
→ Data Coding and Cleaning
→ Regulatory Requirements for CDM
→ Statistical Analysis of Clinical Data
→ Electronic Data Capture (EDC) systems
The duration of the program includes the modules you choose. Connect with your career counselor for the same.
Yes! CliniLaunch offers certification to candidates post clinical data management training completion under PG diploma in clinical research.
The clinical data management program offers a diverse range of career opportunities such as Clinical Data Associate, Clinical Data Coordinator, Clinical Data Manager, Clinical Data Analyst, etc,
Yes, you can pursue clinical research sub-courses in Bangalore online. It will help you learn based on your convenience.
Yes! For financial assistance, connect with your dedicated counselors.