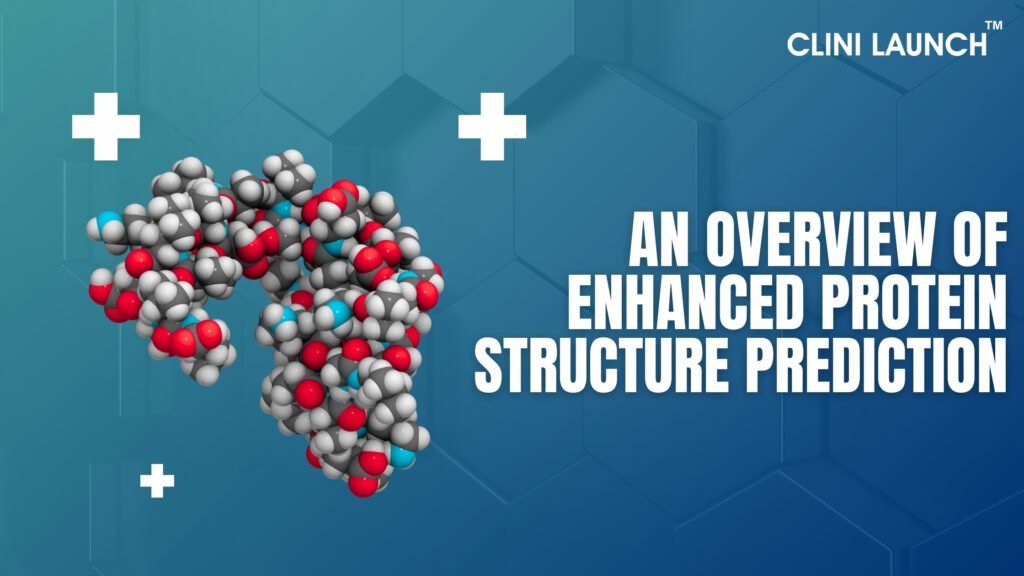
Protein folder problem has a stretch of genomic DNA sequence, by using this, you can predict where the introns are, where transcription will begin and end, where translation will begin and end, and predict distal regulatory elements and methylation sites. With the new protein structure prediction tools, it may change, for this to predict a protein structure and how it can compare to the experimental structure of a reputed structural homolog. This blog outlines an overview of improved protein structure prediction and its definition, approaches, and how it works.
Enroll Now: Bioinformatics course
Understanding Protein Structure Prediction
Large biomolecules from proteins carry out crucial functions within organisms, such as transporting molecules, acknowledging stimuli, offering structuring to cells, and creating metabolic reactions. A protein containing continuous long chains of amino acid linked through peptide bonds. Protein Sequence Analysis usually instant folds into the specific tertiary structure in a natural environment known as native structure where each atom occupies an individual position in the three-dimensional space of the molecule. Through many non-covalent activities, the main factors driving a protein to fold into its native structure are hydrophobic effects, hydrogen bonds, van der Waals forces, and ionic bonds.
In some local regions, protein structures are characterized by a regular conformation shape. The regular, local protein secondary structure is formed by the hydrogen bonds among amide groups of residues. The most frequent secondary structure is the right-handed spiral 𝛼 – helix, in which the supporting amino group donates a hydrogen bond with another backbone carbonyl group, and the structure prediction from sequence distance between these two groups is 3.6 average amino acids. β strand is another common secondary structure which exhibits an almost fully extended shape. Several β parallel or antiparallel strands linked between hydrogen bonds form a β – sheet. For example, which one consists of three α – helices and three β strands, the accurate predicting protein structure of the secondary provides significant information of its tertiary structure.
As protein functions are determined mainly by their tertiary structures, knowledge of the native structures of proteins is highly desirable. Also, experimentally, the native structures of proteins can be used in nuclear magnetic resonance, X-ray crystallography, and cryogenic electron microscopy. Still, there are experimental technologies that are usually costly and time-consuming, and they cannot step up with the quick collection of protein sequences.
On the other hand, this structure determination technologies, the protein structure prediction approaches. For example, protein sequence analysis structure from protein sequences utilizing computing techniques is highly effective. Predicting protein structure purely from its sequence is practical as the structure information is necessary for embedding in the protein sequence. For example, unfolded protein usually refolds to its native structure under conditions when restoring the protein to an aqueous environment.
Approaches and Rationale of Protein Structure Prediction
The precision prediction of protein structures depends heavily on a comprehensive understanding of the protein folding process and the relationship between native structures and protein sequences. The state of native structure of the protein takes the lowest free energy and nearly all extra fit perfectly with their local structural environments.
The evolution history of a query protein, which is normally explained using the multiple sequence alignments (MSAs) of its homologies, offers ample information to gather its native structure. Particularly, the residues with analytic roles in stabilizing structure are partially covered, on the other hand the residues in contact lead to change during the evolutionary process.
In different ways, the protein sequence and structure can be represented. It can also represent the sequences of homology proteins as MSAs or (PSSM) position sequence scoring matrix. Highlighting the correlations among residues for further processing MSAs into hidden profile Markov models or even conditional random fields. Likewise, a protein structure prediction from sequence can be illustrated using the coordinates of all its atoms, the torsion angles related with each Catom, or the distances between residue pairs.
By effectively exploiting the sequence-structure relationship with most of the existing approaches managing structure prediction and the evolutionary information carried by the similar proteins of the target protein. The present approaches can be differentiated into template – based modeling (TBM), which requires template proteins. For example, the proteins with solved structures and free modeling are called ab initio approaches which do not depend on any templates. The TBM approaches can be differentiated into homology modeling and threading.
Protein Structure Prediction Tools Process

Homology-Based Structure Prediction
Protein structure prediction is to balance its amino acid sequence to other protein with a solved structure. This process is called homology-based structure prediction. If the sequences are alike, it stands to reason that their structures should also be similar.
For instance, amino acid sequence homology between the template protein and your protein is comparatively very high, you can simply underlie the side and main chain atoms are known structure of your protein.
A few differences in amino acid sequence, you can underlie the main chain atoms onto these regions and physically determine where the side atoms will end. Once you have an initial model based on sequence homology, you can filter it to ensure that the confirmation things like the bond angles and energy minimization of folds makes theoretical sense.
Threading
Overlaying is not a process in threading, amino acid sequence to a homologous structure, but alternatively you take existing structures and see if your sequence could potentially match their folding. There are so many chances for protein conformations in nature, and even proteins that lack sequence homology to one another may have three-dimensional structures.
For threading, you can pick several candidate templates and utilize them as an algorithm to determine which template results as the best fit, looking at suitable bond angles and the lowest energy score. The process is constant and is a good option if a protein structure with a homologous sequence does not exist.
AlphaFold 2
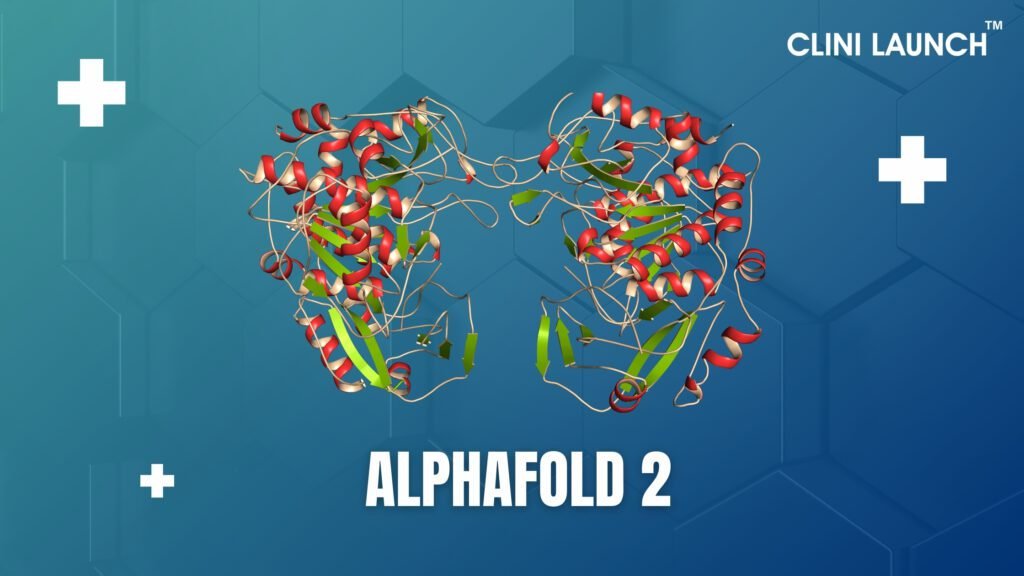
During the 14th critical assessment of critical assessment of structure prediction (CASP14) assessment in 2020. The next approach is made possible by modern computing power and AI is a huge splash. Particularly, DeepMind was co-developed with AlphaFold-2. This method initiated by running a multi-sequence alignment (MSA) that considers the evolutionary relationships between proteins and changes in individual amino acids.
For an example, if a given residue has evolved, then another amino acid paired to that residue will also transform so that the protein’s overall structure is maintained in the variant. The arrangement and pairings are constantly passed through a machine learning algorithm AlphFold-2 is called as an Evo former. This algorithm identifies the best pair interactions and arranges the information to a third portion of the pipeline that creates a structure.
The AlphaFold-2 development team ran the sequences of proteins with experimentally solved structures through the AI pipeline and found that the protein structure prediction was highly like the experimentally determined ones. From the CASP14 challenge, AlphaFold – 2 can predict the harmonization of backbone atoms in space with a precision of 0.96 Å root – mean – square deviation (RMSD) and an all-atom precision of 1.5 Å RMSD. From this aspect, the width of a carbon atom is 1.5 Å, and all atom accuracy of the next best approach entered in CAPS14 was 3.5 Å RMSD.
The deviation of atomic coordinates by less than 1.5 Å would outcome as an actual and predicted structure that are very nearly overlaying upon each other.
Conclusion
The field of protein structure prediction has undergone significant advancements, moving from traditional homology-based and threading methods to revolutionary AI-powered tools like AlphaFold 2. Understanding protein structure is crucial for deciphering its function, and while experimental techniques have limitations in terms of cost and scalability, computational approaches offer a powerful alternative. The development of methods that leverage evolutionary information and machine learning has dramatically improved the accuracy of predictions, bringing us closer to reliably determining the 3D structure of proteins directly from their amino acid sequences. This progress has profound implications for various biological and medical applications, including drug discovery and understanding disease mechanisms.
Ready to delve deeper into the fascinating world of bioinformatics? Enroll in our comprehensive Bioinformatics course at Clinilaunch Research and gain the skills to harness the power of protein structure prediction and other advanced techniques.
Frequently Asked Questions (FAQs)
- What is Protein Structure Prediction?
Protein structure prediction is the process of computationally determining the three-dimensional (3D) structure of a protein based on its amino acid sequence. This is important because a protein’s structure largely dictates its function within a biological system.
- What are the main approaches to Protein Structure Prediction?
The main approaches include:
Homology-Based Structure Prediction: This method builds a model of the target protein based on the known structure of a homologous protein (a protein with a similar sequence).
Threading: This approach involves fitting the amino acid sequence of the target protein onto a library of known protein folds to find the best match.
Ab Initio/Free Modeling: These methods predict the protein structure from first principles, without relying on known structures. AlphaFold 2 is a prominent example of a modern, highly accurate ab initio method.
- How does AlphaFold 2 work?
AlphaFold 2 utilizes a deep learning algorithm that analyzes multiple sequence alignments (MSAs) to understand the evolutionary relationships between proteins and co-evolution of amino acid residues. This information is processed through a neural network architecture called the Evo former, which predicts residue pairings and ultimately generates a highly accurate 3D structure prediction.
- Why is Protein Structure Prediction important?
Knowledge of a protein’s 3D structure is essential for understanding its function, interactions with other molecules, and its role in biological processes. This information is crucial for various applications, including:
Drug Discovery: Identifying potential drug targets and designing molecules that can interact with them.
Understanding Disease Mechanisms: Elucidating how protein misfolding or mutations can lead to diseases.
Biotechnology: Engineering proteins with novel functions for industrial or therapeutic purposes.
- What is the significance of the Root Mean Square Deviation (RMSD) in the context of AlphaFold 2’s accuracy?
The Root Mean Square Deviation (RMSD) is a measure of the average distance between the atoms of a predicted protein structure and the corresponding atoms in the experimentally determined structure. A lower RMSD value indicates a higher degree of accuracy. AlphaFold 2 achieved remarkably low RMSD values (around 0.96 Å for backbone atoms and 1.5 Å for all atoms in some cases), signifying a very high level of agreement between its predictions and experimental data.
References
Protein Structure Prediction: Challenges, Advances, and the Shift of Research Paradigms
https://academic.oup.com/gpb/article/21/5/913/7632861
A Beginner’s Guide to Protein Structure Prediction