Machine learning in healthcare is reshaping how diseases are detected, diagnosed, and treated. Imagine a system where illnesses are caught earlier, treatments become more precise, and patient risks are predicted before they turn critical. That shift is happening because ML models learn from medical data the way doctors learn from experience, but at a scale no human can match. In a recent study, ML-assisted radiology systems showed higher sensitivity and negative-predictive value than radiologists working alone, proving how machine learning strengthens clinical decision-making.
Similarly, a 2022 meta-analysis confirmed that machine-learning models, especially deep-learning architectures, achieved strong diagnostic performance in lung cancer detection using imaging and histopathology data.
These breakthroughs mark a major shift in how healthcare operates. Machine learning is empowering doctors with predictive insights, supporting early diagnosis, accelerating clinical decisions, and personalizing treatment like never before. Instead of reacting to illness, hospitals can now anticipate it creating a smarter, faster, and more life-saving healthcare ecosystem.
All of this brings us to the real question why is machine learning suddenly at the heart of modern healthcare? To understand its growing impact, we need to look at what is driving this massive transformation.
Why Healthcare Depends on ML Today
Healthcare today deals with more data than any human can process scans, lab results, vitals, wearables, EHRs, genetics, everything. Doctors simply don’t have the time to analyze all of them deeply. That’s where machine learning becomes essential. ML can quickly sort through massive data, spot tiny patterns that are easy to miss, and help doctors make decisions faster and with more confidence.
Because of this, ML has sparked some major advancements. It has improved early disease detection, boosted accuracy in reading medical images, predicted patient risks before emergencies happen, and even sped up drug discovery by analyzing molecules in ways humans can’t. It also supports personalized treatment by learning what works best for each type of patient. In short, ML didn’t just assist healthcare; it pushed it into a new level of speed, precision, and prevention.
Before ML vs After ML in Healthcare
| Area | Before ML | After ML |
|---|---|---|
| Diagnosis | Relied heavily on human interpretation; slower; prone to errors | Faster, data-driven, high accuracy; ML supports specialists |
| Medical Imaging | Manual reading; could miss subtle patterns | ML detects patterns invisible to the human eye; earlier detection |
| Treatment Plans | Mostly generalized for all patients | Personalized treatment based on patient-specific data |
| Disease Prediction | Limited predictive ability | predicts risks, complications, and disease progression early |
| Monitoring | Periodic checkups | Continuous real-time monitoring with ML-powered wearables |
| Drug Discovery | Long, expensive, trial-and-error | Faster molecule prediction & drug design |
| Clinical Workflows | High workload, paperwork, delays | Automated documentation, faster decision support |
| Data Analysis | Manual, slow, limited | Instant processing of huge datasets; actionable insights |
How Machine Learning Powers Healthcare: A Step-by-Step Process of Implementation
To appreciate the capabilities of ML in healthcare, we need to understand its workflow from data collection to prediction and the core components that support each stage.
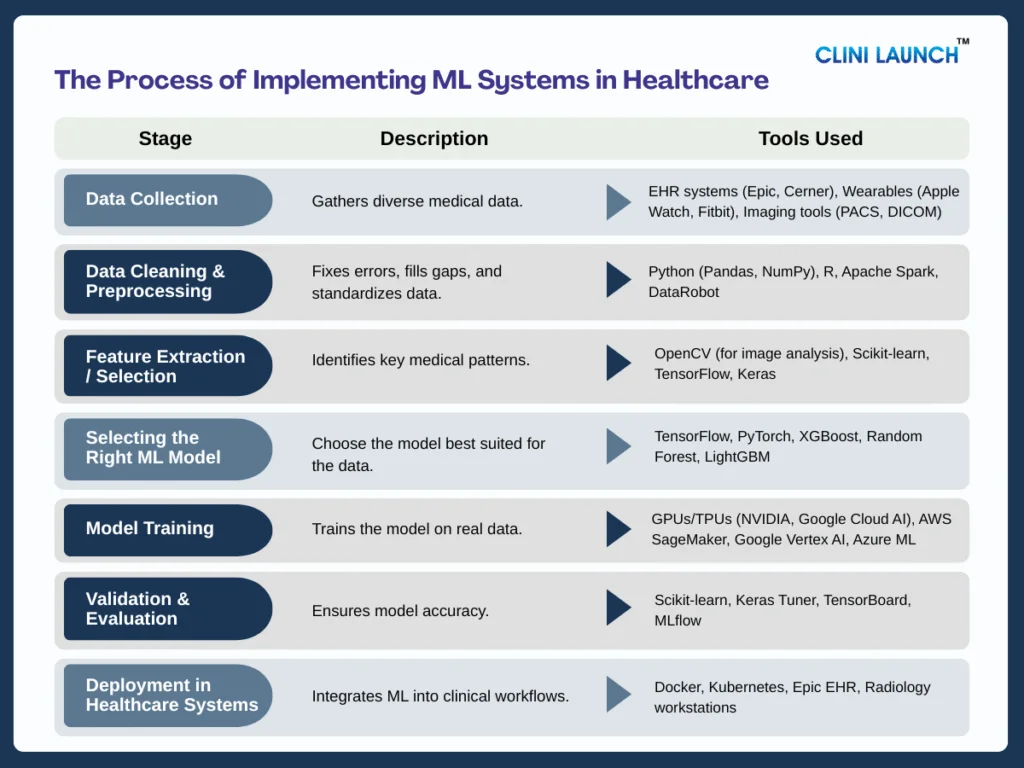
Data Collection
Machine learning relies heavily on high-quality healthcare data, the core component that fuels its ability to learn real medical patterns. This includes scans, EHR entries, lab reports, pathology slides, wearables, and even genomic data for each piece, adding depth to how well the model understands diseases, symptoms, and normal vs abnormal health signals.
In real hospitals, this data flows from imaging centers, ICUs, pathology labs, and patient wearables, creating a rich and diverse dataset that helps ML models view patient health holistically and make more accurate predictions.
| Case Insight: When diabetic patients were not getting specialist eye screenings on time, researchers deployed IDx-DR, an autonomous ML system, directly in primary care clinics. It analyzed retinal images collected on-site and detected diabetic retinopathy with: |
- 87.2% sensitivity
- 90.7% specificity
- 96% usable images
| This showed how collecting real medical images in everyday clinics can power ML to make accurate diagnoses instantly. |
2. Data Cleaning & Preprocessing
This stage relies heavily on the data preprocessing pipeline, the core component responsible for transforming raw medical data into something a model can actually learn from. In healthcare, data is often messy, missing lab values, inconsistent formats, duplicate entries, or device-generated noise. The preprocessing pipeline steps in to clean all of this by fixing errors, filling missing values, removing duplicates, and standardizing formats so the information becomes clear and reliable.
This process may look simple, but it’s one of the most crucial steps in the ML workflow. A model is only as good as the data it learns from, and in clinical settings, even small inconsistencies can lead to incorrect patterns. By turning chaotic patient data into a structured, high-quality dataset, preprocessing ensures that the model starts its learning journey on solid ground.
| Case Insight: TREWS analyzes patient vitals, labs, and EHR trends but only after they are cleaned and standardized through preprocessing pipelines. This allows the ML system to accurately identify early sepsis patterns that humans often miss. When doctors responded to these ML alerts within 3 hours, mortality fell by 3.3% across 5 hospitals. |
3. Feature Extraction / Selection
With clean data in place, the next step is powered by feature engineering, the core component that helps the model identify the most important medical signals. This is where ML goes beyond raw numbers and begins to understand what truly matters whether it’s the sharp edges of a tumor in an MRI, the subtle peaks in an ECG waveform, or unusual trends in a patient’s vitals.
Good feature engineering acts like a spotlight. It highlights the patterns that carry real clinical meaning and dims the noise that could mislead the model. By giving the system the right cues, it ensures the model focuses on the features that doctors actually rely on, setting the stage for more accurate prediction and diagnosis.
| Practical Highlight: ML systems trained on ECG signals can extract subtle features such as heartbeat intervals, waveform peaks, and irregular rhythm patterns. These models often detect atrial fibrillation (AF) more accurately than traditional rule-based methods and they can do it using everyday wearable devices like smartwatches. |
This approach enables early AF detection outside hospitals, making cardiac monitoring more accessible and proactive.
4. Selecting the Right ML Model
Selecting the right ML model is really about choosing how you want the system to think. Each algorithm has its own strengths. CNNs excel at spotting patterns in medical images; NLP models are built to understand clinical notes, and predictive models are great for early-warning alerts or risk scoring. Because healthcare problems vary so much, the choice of model depends entirely on the type of data and the clinical need.
In real teams, Data Scientists experiment with several algorithms, testing what works best, while ML Engineers build and prepare the pipelines needed to train and run them. Clinicians also play a key role here, ensuring the model’s predictions align with real medical reasoning, not just statistical logic.
When this choice is made correctly, the model fits naturally into the clinician’s workflow and improves decision-making without causing friction. But if the wrong model is chosen, even perfectly cleaned and processed data can lead to poor results. This is why understanding the different types of ML models becomes essential for each one is designed to solve a specific kind of healthcare challenge.
To make this clearer, here’s a quick breakdown of the major ML model types and what role each one plays in healthcare:
| Model Type | What It Is | How It Works | Purpose | Used For |
|---|---|---|---|---|
| 1. Supervised Learning | Models are trained with labeled data. | Learn patterns by comparing predictions with correct answers. | Prediction and classification | Disease detection, risk scoring, and outcome prediction. |
| 2. Unsupervised Learning | Models are trained without labels. | Find hidden patterns and clusters in data. | Discovery & Grouping. | Patient segmentation and anomaly detection. |
| 3. Deep Learning | Neural networks with many layers (CNN, RNN, Transformers). | Automatically extracts complex features from images, text, or signals. | High-accuracy pattern recognition. | X-rays, CT/MRI, ECG analysis, clinical text. |
| 4. Reinforcement Learning | Models that learn by trial and error using rewards. | Choose actions → get feedback → improve strategy. | Decision-making. | ICU decisions, treatment optimization, and drug dosing. |
| 5. Probabilistic (Bayesian) Models | Models based on probability and uncertainty. | Updates predictions as new data arrives. | Safe, interpretable predictions. | Disease progression, risk estimation. |
| Practical Highlight: Hospitals use deep convolutional neural networks (CNNs) for lung and breast cancer detection because CNNs outperform traditional ML in recognizing subtle visual patterns in CT and mammography images. |
5. Model Training
Training is the stage where the model actually learns from real patient data. It’s the point where the system evolves from “blank” to “clinically aware,” processing thousands of X-rays, ECG signals, or EHR entries until it begins to recognize meaningful medical patterns. Because healthcare data is massive and complex, this step needs serious compute power GPUs, TPUs, or cloud infrastructure capable of handling huge workloads efficiently.
While the model is learning, Data Scientists fine-tune hyperparameters, adjust learning rates, and rebalance datasets to make sure the model doesn’t overfit or learn the wrong patterns. ML Engineers work in parallel to ensure the entire training pipeline runs smoothly, especially when training is distributed across multiple machines. This is also where challenges like noisy data, missing values, or rare clinical events surface problems that must be handled carefully to keep the model dependable.
By the end of training, the model becomes strong enough to handle real-world patient variability. But this entire process depends heavily on the tools behind the scenes. Different parts of training require different categories of tools from the frameworks used to build models to the compute engines that power them. Here’s a clear breakdown of the key tool categories that make ML training in healthcare possible:
| Category | Tools | Purpose | Who Uses It |
| Deep Learning Frameworks | TensorFlow, PyTorch, Keras | Build and train ML models; define layers, losses, optimizers | Data Scientists, ML Engineers, Researchers |
| Experiment Tracking | MLflow, Weights & Biases, TensorBoard | Log runs, compare models, monitor accuracy/loss | Data Scientists, ML Engineers |
| Data Pipeline Tools | Airflow, Apache Spark, PyTorch DataLoader | Preprocess large datasets, automate workflows | Data Engineers, ML Engineers |
| Compute & Hardware | GPUs, TPUs, Cloud VMs (AWS/GCP/Azure) | Provide the power needed for heavy training | ML Engineers, Cloud Engineers, AI Infrastructure Teams |
| Cloud ML Platforms | AWS SageMaker, Google Vertex AI, Azure ML | Train models at scale without managing servers | ML Engineers, Data Scientists |
| Hyperparameter Tuning | Optuna, Ray Tune, Hyperopt | Auto-optimize model parameters for best performance | Data Scientists |
| Data Storage | AWS S3, GCP Storage, Azure Blob, PACS/DICOM | Store imaging, EHR, and large medical datasets | Data Engineers, ML Engineers |
| Case Insight: DeepMind trained a model on 703,782 VA patient records Using large-scale GPU infrastructure. The trained model predicted: |
- 55.8% of AKI cases 48 hours early
- 90.2% of severe kidney injury cases requiring dialysis
This shows how high-performance computing enables powerful predictive healthcare models.
6. Validation & Evaluation
Before any ML model is deployed in a hospital, it must undergo strict validation to prove it is safe, reliable, and trustworthy. Healthcare uses rigorous evaluation of metrics sensitivity, specificity, precision, recall, F1-score, and AUC because patient safety depends on accuracy in real situations. Sensitivity ensures serious conditions aren’t missed, while specificity prevents unnecessary alerts that overwhelm doctors.
Validation teams test the model on completely unseen patient data to make sure it generalizes well beyond its training set. They also evaluate its performance across different hospitals, devices, demographic groups, and even edge cases to ensure consistency.
Clinical researchers, regulatory bodies, data scientists, and QA teams collaborate during this stage to confirm that the model behaves responsibly in real clinical environments. Many models fail validation, and that’s normal for this step to function as the final safety gate before a model is allowed anywhere near real patients.
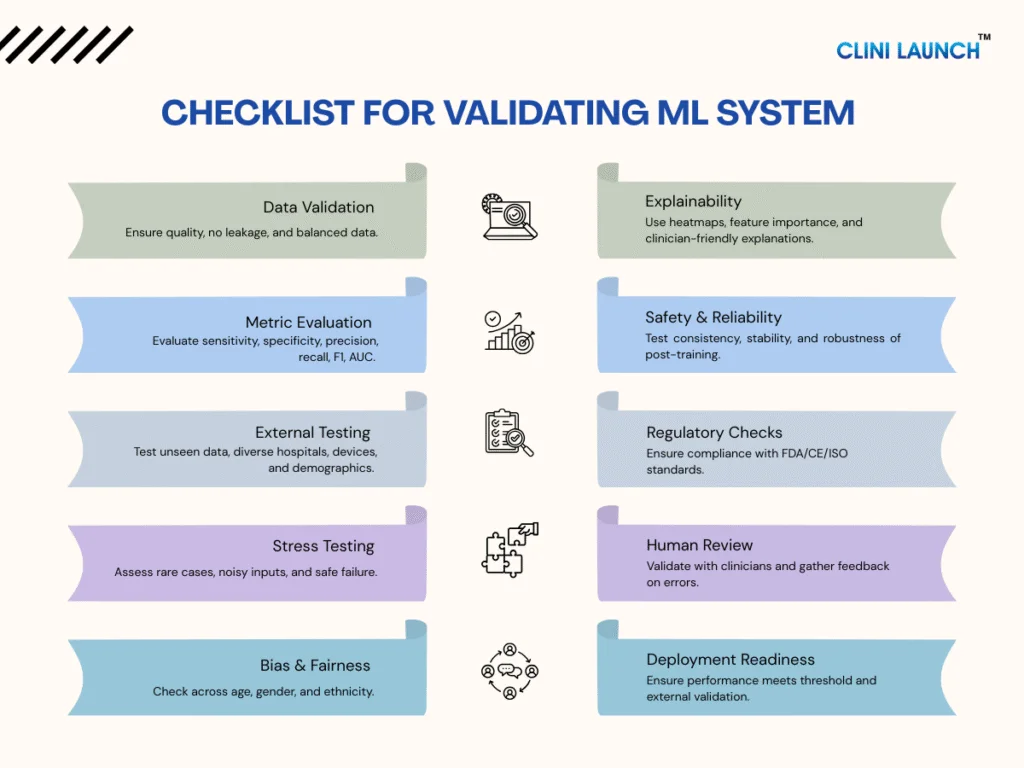
To manage all these checks systematically, teams follow a structured validation checklist. This helps make sure every part of the model for its data, metrics, fairness, safety, and regulatory readiness is thoroughly reviewed before deployment. Here’s a quick breakdown of what that checklist looks like:
| Area | Key Checks |
|---|---|
| Data Validation | Quality check, no data leakage, balanced groups, multi-hospital data |
| Metric Evaluation | Sensitivity, specificity, precision, recall, F1-score, AUC |
| External Testing | Unseen data, new hospitals, different devices, demographic performance |
| Stress Testing | Rare cases, noisy inputs, edge conditions, safe failure behavior |
| Bias & Fairness | Compare results across age, gender, ethnicity |
| Explainability | Heatmaps, feature importance, clinician-friendly explanations |
| Safety & Reliability | Consistent outputs, stability after retraining, robustness |
| Regulatory Checks | Documentation, experiment logs, compliance (FDA/CE/ISO) |
| Human Review | Clinician validation, feedback on errors, workflow fit |
| Deployment Readiness | Meets performance threshold, passes external validation |
| Practical Highlight: Before deployment, AI systems for chest X-rays (e.g., pneumonia detection tools) undergo validation using held-out test data. Metrics like AUC (0.94+), sensitivity, and specificity determine whether the model is accurate enough for real clinical use. |
7. Deployment in Healthcare Systems
After a model passes validation, the next step is getting it into the hands of clinicians and this relies on deployment platforms, the core component that brings ML into real medical workflows. These platforms integrate the model directly into tools doctors already use, like radiology workstations, EHR dashboards, clinical apps, or even patient wearables.
The goal here is simple: deliver predictions in a clear, actionable, and seamless way. Whether it’s highlighting abnormalities on a scan or displaying a risk score inside a patient’s chart, deployment platforms ensure the model’s intelligence becomes part of everyday clinical decision-making without disrupting the existing flow of work.
| Practical Highlight: Many hospitals using the Epic EHR system rely on the Epic Sepsis Model (ESM)—a machine-learning-based risk scoring tool that continuously analyzes patient vitals, labs, and clinical data already stored in the EHR. The model updates sepsis risk scores automatically throughout the day, displaying alerts directly inside the patient’s chart. This allows clinicians to receive early warnings without switching screens or using additional software, improving workflow adoption, and supporting faster, real-time decision-making. |
8. Prediction & Real-Time Decision Support
At this stage, the model finally steps into real clinical action, powered by the inference engine the core component that enables instant, real-time predictions. As new patient data flows in, the inference engine processes it within seconds, allowing the system to detect abnormalities, forecast patient deterioration, or even suggest possible treatment steps right when they’re needed at most.
This real-time intelligence is what makes ML truly valuable at the bedside or in the emergency room. Instead of waiting for manual review or delayed analysis, clinicians receive immediate insights that support faster, more informed decision-making during critical moments of patient care.
| Practical Highlight: ML systems deployed in emergency departments can analyze head CT scans within seconds, flagging early signs of brain hemorrhage and immediately alerting radiologists. This rapid, automated detection helps reduce treatment delays and speeds up critical stroke intervention, especially when every minute matters. |
9. Continuous Learning & Improvement
Even after deployment, the model’s journey isn’t over. Its growth is driven by the feedback loop, the core component that allows ML systems to keep learning from real clinical outcomes and day-to-day doctor interactions. Every corrected prediction, every mislabeled scan, and every new patient case becomes valuable information that helps the model evolve.
Through this continuous flow of feedback, the model adapts to new patterns, refines its predictions, and steadily becomes more accurate and reliable over time. In a field as dynamic as healthcare, this ability to learn from real-world practice is what ensures ML stays relevant, up-to-date, and increasingly aligned with clinical needs.
| Case Insight: Radiologists working with AI-assisted breast cancer screening systems often catch more subtle cancers than when working alone. A 2025 multicenter study demonstrated that combining radiologist judgment with AI-CAD significantly improved cancer detection rates while reducing reader workload. This shows how real-world clinical feedback and human-AI collaboration strengthen the accuracy and reliability of breast cancer screening tools. |
How ML Training Gives Students a Massive Career Boost in Healthcare
Machine learning is revolutionizing healthcare, and students who master both medical data and ML are becoming some of the most sought-after professionals today. By gaining hands-on experience with real-world datasets like EHRs, MRI scans, and lab results, students can directly contribute to healthcare advancements. These are the same data used by hospitals, health-tech companies, and diagnostics startups to detect diseases earlier, analyze medical images, and predict patient risks with remarkable accuracy.
This unique combination of medical knowledge and technical skills opens the door to high-impact roles such as Healthcare Data Scientist, AI Radiology Analyst, Bioinformatics Specialist, Clinical Data Engineer, and Medical AI Product Designer. These roles aren’t just impactful; they’re also highly rewarding. The demand for these roles is rising fast, with the U.S. Bureau of Labor Statistics projecting a 15% growth in data science jobs from 2022 to 2032
The demand is only set to rise. The global market for AI in healthcare is expected to skyrocket from USD 11.7 billion in 2023 to USD 188.5 billion by 2030, making this a rapidly expanding field
As AI becomes critical in diagnostics, drug discovery, clinical automation, and wearables, students trained in ML are in prime position for future-ready, high-demand roles. The opportunities are growing fast, and the healthcare sector needs professionals who can blend medical expertise with machine learning to drive innovation forward.
Conclusion
Machine Learning is transforming healthcare through early detection, smarter decisions, and more precise treatments, and students who learn ML today will be the innovators leading tomorrow’s medical breakthroughs. If you want to be part of the future of healthcare, now is the right time to begin. CliniLaunch Research Institute offers a specialized AI & ML in Healthcare course designed to equip aspiring professionals with the skills needed to thrive in this rapidly evolving field.