The Clinical Data Coordinator is one of the most critical entry-level roles in clinical research for anyone aiming to build a non-laboratory career in healthcare. As clinical trials become increasingly data-driven, multi-site, and tightly regulated, this role exists to protect one thing the industry cannot afford to lose: data integrity.
Every clinical trial generates massive volumes of patient data. That data must be accurate, consistent, traceable, and compliant with global regulatory standards. If it is not, the trial risks delays, audit findings, inspection observations, or outright failure. The clinical data coordinator plays a central role in preventing those outcomes by ensuring trial data is review-ready, compliant, and reliable throughout the study lifecycle.
For students exploring a career in clinical research, understanding this role is often the first practical step toward entering the industry without working at the lab bench.
Who is a Clinical Data Coordinator?
A Clinical Data Coordinator (CDC) is a clinical research professional who supports clinical data management activities during a clinical trial. This role does not generate patient data. Instead, it focuses on reviewing, validating, and coordinating data collected from clinical sites to ensure it meets protocol, quality, and regulatory requirements.
CDC works closely with clinical research associates, investigators, site teams, and data managers. Their job is to make sure data entered into electronic data capture systems is accurate, complete, consistent, and aligned with the study protocol.
In practical terms, this role sits between data collection at the site level and centralized data management. By coordinating data flow across teams and systems, the clinical data coordinator ensures that issues are identified early, queries are handled properly, and patient safety data remains consistent across the trial.
For beginners, this role offers structured exposure to how real clinical trials operate from a data quality, compliance, and regulatory perspective, making it a common and logical entry point into clinical research careers.
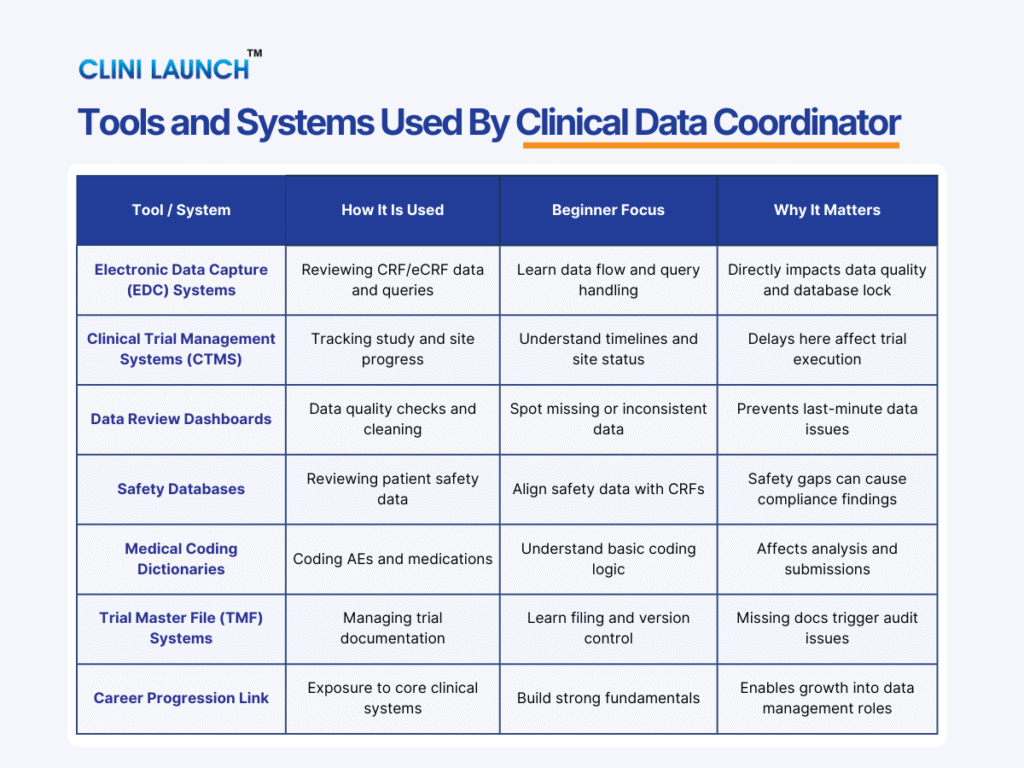
Core Responsibilities of a Clinical Data Coordinator
The core responsibilities of a Clinical Data Coordinator focus on ensuring that clinical trial data is accurate, complete, traceable, and compliant throughout the study lifecycle. This role supports the smooth flow of data from clinical sites to central databases while maintaining regulatory alignment and database readiness.
Below is a clear and beginner-friendly explanation of clinical data coordinator roles and responsibilities, supported by real-world, industry-based examples.
1.Supporting Clinical Data Management Activities
Clinical trials follow a predefined data management plan that defines how data should be collected, reviewed, cleaned, and locked. The Clinical Data Coordinator supports this plan by ensuring that daily data workflows are executed consistently across the study. This includes coordinating data review cycles, tracking data-related issues, and working closely with central data management teams to resolve inconsistencies early rather than at the end of the trial.
2.Case Report Forms and eCRF Handling
Case report forms are the primary tools used to capture patient data in a clinical trial. In most modern studies, this data is entered through electronic case report forms using electronic data capture systems. The Clinical Data Coordinator ensures that these forms are completed accurately and in line with the approved protocol. This involves reviewing form completeness, verifying required fields, coordinating updates after protocol amendments, and supporting corrections when inconsistencies are identified. Proper CRF and eCRF handling are essential for maintaining data consistency and accuracy across study sites.
Clinical Research
Build practical, industry-aligned skills to work across real clinical trial environments. Learn how clinical studies are planned, conducted, documented, and monitored, with a strong emphasis on ethics, patient safety, and regulatory compliance throughout the trial lifecycle.

Duration: 6 months
Skills you’ll build:
3.Data Entry and Validation Support
Although patient data is entered by clinical sites, the Clinical Data Coordinator plays a key role in reviewing and validating that data before it progresses through the data lifecycle. This includes checking entries in electronic data capture systems for missing, illogical, or inconsistent values and supporting structured data entry and validation of workflows. When recurring issues are identified, they are escalated to data managers to prevent systemic data quality problems. These activities contribute directly to early-stage data cleaning in clinical trials.
4.Query Management and Data Cleaning
When discrepancies or missing information are identified during data review, data queries are raised for clarification. The Clinical Data Coordinator supports query management by reviewing system-generated and manual queries, coordinating responses with site teams, and tracking query resolution status. Active involvement in ongoing data cleaning helps prevent query backlogs and reduces delays during database locks.
In a multi-site Phase III clinical trial, data queries were raised regularly for missing and inconsistent entries, but follow-ups with sites were delayed. As the study approached database lock, unresolved queries accumulated, slowing final data cleaning and putting the lock timeline at risk.
The Clinical Data Coordinator intervened by prioritizing high-impact queries, coordinating closely with sites and Clinical Research Associates, and enforcing structured tracking with clear timelines. This focused approach cleared the backlog, enabled database lock on schedule, and prevented delays to final analysis and regulatory activities.
Regulatory guidance from the U.S. FDA emphasizes timely data review, correction of discrepancies, and readiness of electronic source data before database lock and submission.
5.Source Data Verification Support
During monitoring visits, Clinical Research Associates perform source data verification by comparing site source documents with data entered the clinical database. The Clinical Data Coordinator supports this process by clarifying discrepancies raised during monitoring, coordinating corrections with sites, and ensuring related documentation is updated correctly. This support strengthens audit and inspection of readiness and improves overall trial quality.
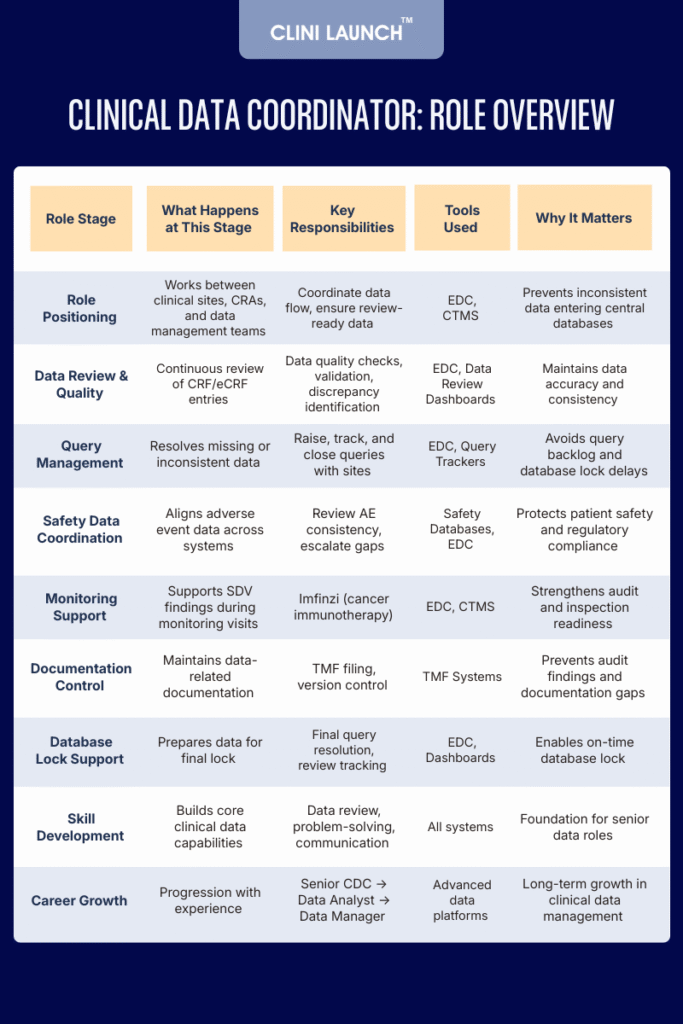
6. Patient Safety Data Coordination
Patient safety data must be accurate, timely, and consistent across systems to meet regulatory expectations. The Clinical Data Coordinator supports patient safety data coordination by verifying adverse event entries, ensuring consistency between safety databases and case report forms, escalating missing or delayed safety data, and aligning safety information with regulatory reporting timelines.
During routine data review in an ongoing clinical trial, inconsistencies were identified between adverse events recorded in case report forms and entries in the safety database. If left unresolved, these discrepancies could have resulted in compliance findings during regulatory inspection.
The Clinical Data Coordinator reviewed safety data entries, coordinated corrections with study sites, and ensured alignment between clinical and safety systems. As a result, patient safety data became consistent across platforms; regulatory reporting timelines were met, and inspection risk was reduced.
Regulatory inspection trend reports published by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) frequently cite safety data inconsistencies and documentation gaps as inspection observations.
7. Regulatory Compliance and ICH GCP Alignment
All clinical trial data activities must align with ICH GCP compliance and applicable regulatory requirements. The Clinical Data Coordinator ensures protocol adherence during data review, proper documentation for audits, and compliance with regulatory expectations. The coordinator also supports sponsor reviews and inspections by maintaining inspection-ready data and documentation throughout the trial.
8.Trial Master File and Documentation Support
Proper documentation is essential for trial transparency and regulatory inspections. The Clinical Data Coordinator supports trial master file activities by ensuring that data-related documents are filed correctly, version control is maintained, and documentation practices remain inspection-ready throughout the study.
During a sponsor audit, missing data review documentation was identified for several completed study visits. Although the clinical data itself was accurate, incomplete documentation raised concerns about data traceability and inspection readiness.
The Clinical Data Coordinator reviewed filing practices, ensured missing documents were added to the trial master file, and standardized documentation workflows. The audit observations were resolved successfully, and improved documentation practices were applied to ongoing and future studies.
Regulatory inspection summaries published by the U.S. Food and Drug Administration (FDA) consistently highlight documentation gaps and incomplete records as common audit findings in clinical trials.
9.Database Lock Support
Before a clinical trial database can be locked, all data queries must be resolved, and final data reviews completed. The Clinical Data Coordinator supports database lock activities by tracking outstanding queries, confirming site responses, supporting final review cycles, and coordinating with data managers prior to lock approval. This ensures that the database is complete, accurate, and ready for submission.
10.Medical Coding Coordination
Some clinical trials require standardized coding of medical terms such as adverse events and medications. The Clinical Data Coordinator assists in medical coding coordination by supporting review of coded terms, coordinating corrections when inconsistencies are identified, and ensuring consistency across datasets used for analysis and reporting.
11. Collaboration with Clinical Teams
Clinical trials depend on effective coordination across multiple teams. The Clinical Data Coordinator works closely with Clinical Research Associates, investigators, site staff, and data managers to resolve data-related issues, support site communication, and ensure smooth operational flow throughout the trial.
Together, these responsibilities ensure that clinical trial data is reliable, audit-ready, and suitable for regulatory submission, reinforcing the Clinical Data Coordinator’s role as a critical link between clinical sites and data management teams.
Key Skills and Career Growth for a Clinical Data Coordinator
| Key Skill Area | What It Enables in the Role | How This Skill Is Built | Career Growth It Supports |
|---|---|---|---|
| Attention to Detail & Data Review | Identifying missing, inconsistent, or incorrect data | Regular data review, query checks, and CRF verification | Progression to Senior Clinical Data Coordinator |
| Clinical Data Management Knowledge | Working with CRFs, eCRFs, and data review cycles | Exposure to data management plans and review workflows | Transition into Clinical Data Analyst or Data Manager |
| Regulatory & ICH GCP Awareness | Ensuring compliant data handling and audit readiness | Working with protocols, GCP guidelines, and inspections | Eligibility for lead and compliance-focused roles |
| Query Management & Issue Resolution | Coordinating with sites and CRAs to close data queries | Handling live queries and site clarifications | Study-level ownership and senior coordinator roles |
| Problem-Solving Ability | Identifying data issues and driving corrective actions | Managing discrepancies and recurring data issues | Readiness for complex trials and lead roles |
| Communication & Coordination | Working with investigators, CRAs, and data teams | Daily interaction with cross-functional teams | Growth into Clinical Data Operations roles |
| Documentation & Process Tracking | Maintaining inspection-ready records | Managing trackers, TMF documents, and audit files | Supports management and oversight responsibilities |
Clinical SAS
Build practical skills in clinical data analysis and statistical reporting using SAS, aligned with regulatory standards used in clinical trials. Learn how clinical trial data is structured, analyzed, and converted into submission-ready outputs.

Duration: 6 months
Skills you’ll build:
Conclusion
The Clinical Data Coordinator role exists to keep a clinical trial under control. Modern trials involve multiple sites, large volumes of patient data, and strict regulatory oversight. When data is not reviewed, coordinated, and resolved in real time, problems surface late, during audits, inspections, or database lock. This role prevents that by acting as the link between sites, clinical teams, and data management.
For beginners, the value of this role is exposure. You see how trials actually function, how data flows, where mistakes happen, and how those mistakes are corrected before they become regulatory issues. You are not isolated in one task; you are embedded in the operational backbone of a trial.
To enter and succeed in this role, theoretical knowledge alone is not enough. What matters is practical familiarity with clinical trial workflows and regulatory expectations. Structured, industry-aligned training helps bridge that gap and prepares candidates to operate confidently in real clinical research environments.
Clini Launch Research Institute offers an industry-aligned clinical research course that equips learners with the skills and hands-on exposure required to confidently begin a career as a Clinical Data Coordinator and grow within the clinical research industry.
Frequently Asked Questions (FAQs)
1. What does a Clinical Data Coordinator do?
A Clinical Data Coordinator reviews and coordinates clinical trial data to ensure it is accurate, complete, and compliant with study protocols and regulatory guidelines.
2. Is the Clinical Data Coordinator role suitable for beginners?
Yes. It is an entry-level role in clinical research and is suitable for life science graduates who want a non-laboratory career path.
3. Does a Clinical Data Coordinator perform data entry?
No. Data is entered by clinical sites. The coordinator reviews, validates, and resolves data issues.
4. What tools does a Clinical Data Coordinator work with?
They commonly work with electronic data capture systems, clinical trial management systems, safety databases, and trial master file systems.
5. What is the career growth after becoming a Clinical Data Coordinator?
With experience, professionals can progress into Senior Clinical Data Coordinator, Clinical Data Manager, or Clinical Data Operations roles.
6. How can I prepare for a Clinical Data Coordinator role?
Preparation involves understanding clinical research processes, basic data management concepts, regulatory guidelines, and gaining practical exposure through structured training.







