A Clinical Research Coordinator (CRC) supports the execution of clinical trials at the study site. They help coordinate patient visits, manage study documents, support informed consent, assist with data collection, and ensure the study follows the approved protocol and regulatory guidelines.
Imagine a hub where scientific protocols, patient care, documentation, sponsor expectations, and compliance all intersect—that’s where a Clinical Research Coordinator (CRC) comes in. CRCs are the operational heart of clinical studies, ensuring that trials are conducted ethically, efficiently, and in strict accordance with regulations and protocols. Clinical trials are the backbone of modern medicine, and behind every successful trial is a skilled professional managing clinical research coordinator roles and responsibilities to keep studies on track.
Research on clinical trial workforce trends shows that the number of registered clinical trials has increased by over 30%, leading to a growing demand for skilled coordinators. This rise highlights the critical role CRCs play in managing trial complexity and supporting timely, high-quality research outcomes.
In this blog, you’ll discover Clinical Research Coordinator Roles and Responsibilities, what they do every day, and how they manage critical aspects like patient visits and study coordination. Whether you’re a student considering a career in clinical research, a team lead wanting to understand your CRC better, or a manager seeking to optimize your study operations, this guide offers practical clarity on clinical research coordinator roles and responsibilities.
Who is clinical Research coordinator?
A Clinical Research Coordinator (CRC) supports the daily conduct of a clinical trial at the study site. They coordinate study activities, manage documentation, and ensure procedures are followed according to the approved protocol. While the principal investigator oversees medical decisions, the CRC handles site-level coordination, so the trial runs smoothly and in compliance.
CRCs work closely with investigators, study staff, sponsors, and the ethics committee to keep communication clear and timely. They help track study timelines, support patient screening and recruitment, and maintain records, so data remains accurate and audit-ready an essential part of CRC responsibilities in clinical trials.
By managing documentation flow, regulatory requirements, patient coordination, and day-to-day trial activities, the clinical research coordinator roles and responsibilities are central to consistent trial conduct and data accuracy. In many research settings, especially within CRC role in hospitals, CRCs support safety reporting compliance and essential documents maintenance, strengthening overall site operations. Evidence from clinical research settings shows that over 80% of sites report improved trial quality and execution when supported by a dedicated CRC, highlighting the importance of effective job role execution and strict ICH-GCP compliance at the site level. This highlights the importance of the clinical research coordinator job role in maintaining operational control and regulatory alignment at the study site.
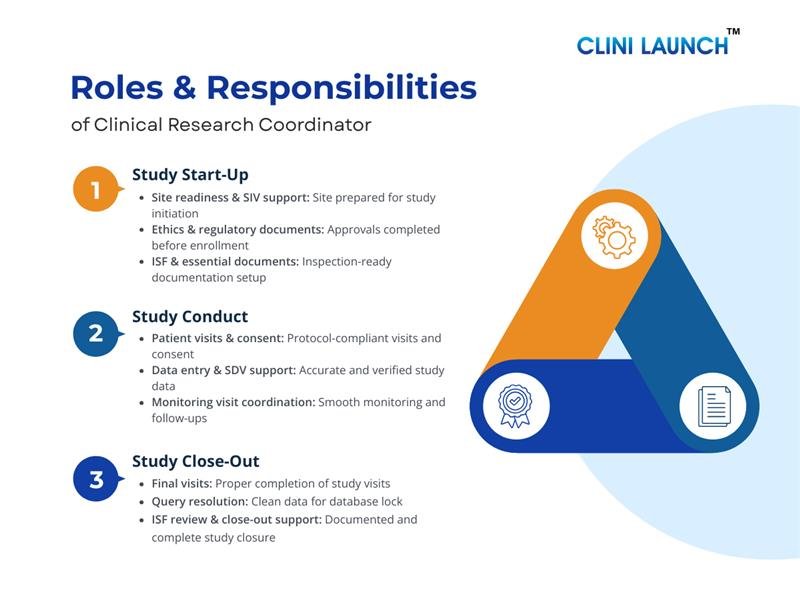
Roles and Responsibilities of a Clinical Research Coordinator
This section explains the clinical research coordinator roles and responsibilities across study start-up, conduct, and close-out phases, reflecting real-world expectations outlined in a standard clinical research coordinator for job description.
Phase 1: Study Start-Up Phase
The study start-up phase prepares the site before enrollment begins. During this stage, the CRC organizes site readiness and ensures alignment with protocol and compliance requirements.
Site Readiness and Study Initiation Support
The CRC supports site readiness by coordinating internal activities, organizing study materials, and assisting with preparation for the Site Initiation Visit (SIV). This ensures the site understands study workflows and is operationally prepared before the trial begins.
Regulatory and Ethics Documentation Support
During start-up, the CRC assists with regulatory and ethics documentation by compiling required records, tracking submission status, and maintaining approval-related correspondence. This helps ensure that all necessary approvals are in place before participant enrollment.
Essential Study Documentation Setup
CRCs organize the trial master file, ISF, delegation logs, training records, and approvals—ensuring inspection of readiness from day one. This ensures documentation is complete, current, and inspection-ready from the start of the study.
CRC Responsibilities During the Study Start-Up Phase
| CRC Activity | Purpose | What It Ensures |
|---|---|---|
| Site readiness and study initiation support | Prepare the site before trial initiation | Operational readiness and clear workflows |
| Regulatory and ethics documentation support | Complete required submissions and approvals | Compliance before participant enrollment |
| Essential study documentation setup | Organize ISF and key study records | Inspection-ready documentation |
Case Study: Impact of Protocol Deviations on Clinical Trial Quality
| What Happened | Impact | Why CRC Matters |
|---|---|---|
| Clinical research literature shows that protocol deviations—departures from the approved study procedures— frequently occur during the active conduct phase of clinical trials and can compromise both participant safety and the reliability of trial data. These deviations may include missed assessments, improper documentation, or procedures conducted outside the defined protocol. | When protocol deviations are not properly identified, recorded, and managed, they can undermine the scientific validity of a trial, affect data integrity, and potentially jeopardize patient welfare. This can lead to increased monitoring findings, longer trial completion times, and difficulties during regulatory review if deviations are widespread or poorly documented. | During the study conduct phase, Clinical Research Coordinators (CRCs) help minimize protocol deviations by ensuring daily trial activities strictly follow the approved protocol, supporting accurate record-keeping, and coordinating with monitors and investigators to address discrepancies quickly. Their ongoing oversight is key to maintaining data quality and overall study integrity. |
Phase 2: Study Conduct Phase
CRCs support active trial execution and ensure ongoing compliance throughout the conduct phase. CRCs also support the clinical research coordinator for job description by assisting with adverse event reporting and serious adverse event reporting in coordination with the investigator and sponsor.
Patient Visit Coordination and Study Activities
The CRC coordinates participant visits according to the study schedule by arranging appointments, preparing visit-related materials, and supporting site staff during study procedures. This helps ensure visits are conducted on time and in line with protocol requirements.
Informed Consent and Participant Support
CRCs support the informed consent process and informed consent documentation, ensuring ethical participation and compliance. The CRC also assists with participant communication to support adherence to visit schedules and study requirements.
Data Collection and Documentation Maintenance
CRCs maintain source documentation, complete case report forms, and support data entry and data accuracy. This helps maintain data quality and consistency throughout the study.
Monitoring Visit and Sponsor Coordination
CRCs support monitoring visit support, SDV activities, and maintain audit and inspection of readiness, ensuring study documents are available for review, and addressing follow-up actions. This helps maintain ongoing oversight and inspection of readiness during the study.
CRC Responsibilities During the Study conduct Phase
| CRC Activity | Purpose | What It Ensures |
|---|---|---|
| Patient visit coordination and study activities | Manage scheduled study visits and protocol-required procedures | Protocol-compliant and timely study visits |
| Informed consent and participant support | Maintain valid, informed, and ongoing consent | Ethical participant involvement and regulatory compliance |
| Data collection and documentation maintenance | Record, verify, and manage study data accurately | Data quality, traceability, and consistency |
| Monitoring visit and sponsor coordination | Support sponsor monitoring, audits, and oversight activities | Inspection readiness and timely issue resolution |
| What Happened | Impact | Why CRC Matters |
|---|---|---|
| During the conduct phase of clinical trials, loss to follow-up occurs when enrolled participants fail to return for scheduled study visits or withdraw prematurely. Clinical research methods literature indicates that approximately 20% of subjects may be lost to follow-up, which can introduce bias and misleading results, particularly when missing outcomes differ between treatment groups. | High rates of loss to follow-up can distort treatment effect estimates, threaten the internal validity of trial results, and necessitate additional statistical adjustments or sensitivity analyses. These issues complicate data interpretation and may raise concerns during regulatory review and acceptance. | Clinical Research Coordinators (CRCs) play a key role in minimizing loss to follow-up by maintaining participant engagement, proactively tracking visit schedules, following up with participants, and documenting reasons for missed visits. Their efforts help protect data completeness, reduce bias, and preserve the overall quality and validity of trial data. |
Phase 3: Study Close-Out Phase
The study close-out phase begins after the last participant completes the final study visit and continues until all study activities at the site are formally completed. During this stage, the Clinical Research Coordinator (CRC) supports the completion of site-level activities by ensuring documentation is finalized, data is resolved, and the site is ready for study closure and potential inspections.
Final Patient Visit and Study Completion Support
The CRC supports final study visits by coordinating end-of-study assessments, ensuring required procedures are completed, and confirming that participant records are properly closed. This helps ensure that all subject-related activities are completed in line with the protocol.
Data Cleaning and Query Resolution
During close-out, the CRC supports data cleaning by responding to outstanding data queries, verifying source documents, and assisting with final Case Report Form (CRF) completion. This helps ensure that data is accurate, complete, and ready for database locking.
Essential Document Review and Archival Preparation
The CRC reviews the Investigator Site File (ISF) to ensure all required documents are complete, current, and properly filed. This includes confirming approvals, correspondence, and study records are ready for long-term storage according to regulatory requirements ensure records meet essential documents of maintenance standards.
Close-Out Visit and Sponsor Coordination
The CRC supports site close-out visits by coordinating with Clinical Research Associates (CRAs), making documents available for review, and addressing close-out findings. This helps ensure that all site responsibilities are formally completed and documented.
CRC Responsibilities During the Study Close-Out Phase
| CRC Activity | Purpose | What It Ensures |
|---|---|---|
| Final patient visit coordination | Support completion of end-of-study and follow-up visits | Proper subject closure in accordance with the protocol |
| Data cleaning and query resolution | Address outstanding data issues and resolve queries | Readiness for database lock and analysis |
| Essential document review (ISF) | Verify completeness and accuracy of study documentation | Inspection-ready study records |
| Close-out visit support and coordination | Assist the CRA during site close-out activities | Formal completion of site-level study activities |
| What Happened | Impact | Why CRC Matters |
|---|---|---|
| The U.S. FDA issued a warning letter to a clinical investigator for failing to retain required study records for the mandated retention period. Record retention is a critical responsibility during the study close-out and archiving phase, and failure to meet these requirements represents a significant regulatory non-compliance. | Inadequate or missing record retention raises serious concerns regarding the validity, reliability, and integrity of site-level trial data. Such deficiencies can result in corrective and preventive actions (CAPAs), heightened regulatory scrutiny, and potential restrictions on future research activities. | During the close-out phase, Clinical Research Coordinators (CRCs) help prevent record retention failures by reconciling essential documents, ensuring proper archiving, maintaining document traceability, and confirming that records remain accessible for inspections throughout the required retention period. Their oversight helps keep the site inspection-ready even after trial completion. |
Skills Required for a Clinical Research Coordinator (CRC)
A Clinical Research Coordinator requires a strong foundation in clinical research and technical skills to support compliant trial execution. This includes understanding clinical trial protocols, ICH-GCP compliance, informed consent handling, source data verification, and proper management of essential study documents such as the Investigator Site File (ISF). These skills ensure trials are conducted ethically, data remains accurate, and sites remain inspection-ready throughout the study’s lifecycle.
In addition to technical knowledge, CRCs need operational and professional skills to manage daily trial activities effectively. This includes coordinating study visits, working closely with investigators, CRAs, and study teams, resolving data queries, tracking timelines, and maintaining clear communication. Strong attention to detail, time management, and ethical awareness enable CRCs to handle multiple responsibilities while maintaining compliance and consistent trial performance.
Conclusion
A CRC’s daily work directly affects trial quality, patient safety, and data integrity. From documentation to coordination, clinical research coordinator roles and responsibilities are central to ethical and efficient research execution. Every task, whether managing patient visits, maintaining documentation, or supporting compliance, directly affects the quality, safety, and credibility of a clinical trial. This makes the CRC role essential to ensuring that research studies are conducted responsibly and efficiently at the site level.
For those exploring a career in clinical research, understanding the CRC role provides clarity on what real trial execution looks like beyond theory. To learn more about this role in depth, explore the skills that define the CRC blog on the CliniLaunch website, and for hands-on, job-ready training, consider enrolling in the Advanced Diploma in Clinical Research to build the skills required to succeed in this field.
FAQ
1. What are the roles and responsibilities of a Clinical Research Coordinator?
A Clinical Research Coordinator supports the day-to-day conduct of clinical trials at the site. They coordinate study activities, manage documentation, support patient visits, and ensure the trial follows the approved protocol and GCP requirements.
2. What does a Clinical Research Coordinator do in a clinical trial?
A CRC coordinates patient visits, maintains study records, supports data entry, and works with investigators and monitors to keep the trial running smoothly and compliantly throughout its lifecycle.
3. What is the difference between a CRC and a CRA?
A CRC works at the study site handling daily trial execution, while a CRA works for the sponsor or CRO to monitor sites, review data, and ensure compliance across multiple sites.
4. What is the difference between a CRC and a CRA?
A CRC typically reports to the Principal Investigator (PI) at the site and works closely with Clinical Research Associates (CRAs) for study of coordination and monitoring activities.
5. What qualifications are required to become a Clinical Research Coordinator?
Most CRCs have a background in life sciences, pharmacy, nursing, or healthcare. Formal training in clinical research and GCP knowledge significantly improves eligibility and readiness for the role.
6. What skills are needed for a Clinical Research Coordinator?
CRCs need strong protocol understanding, documentation skills, attention to detail, coordination ability, and clear communication skills to manage study activities and compliance effectively.
7. What is the role of a CRC in patient safety?
CRCs support patient safety by ensuring informed consent is properly documented; adverse events are reported on time, and study procedures are followed as per protocol and ethical guidelines.
8 . Does a Clinical Research Coordinator take informed consent?
A CRC supports the informed consent process by explaining study details and documenting consent, but the investigator retains final responsibility for ensuring consent is ethically and medically appropriate.
9. Is Clinical Research Coordinator a good career option?
Yes, the CRC role offers hands-on exposure to clinical trials, strong demand across hospitals and research sites, and a solid foundation for long-term growth in clinical research.
10 . What is the career growth path for a Clinical Research Coordinator?
With experience, CRCs can progress to roles such as Senior CRC, CRA, Clinical Trial Manager, or Project Coordinator, depending on skills, exposure, and career interests.







