A Clinical Trial Assistant (CTA) is one of the most sought-after entry-level roles for research aspirants. This position focuses on administrative and operational responsibilities, such as documenting, drafting, and maintaining the essential records that clinical trials rely on. CTAs work closely with coordinators, investigators, and project managers to ensure accuracy, regulatory compliance, and smooth communication across all study sites. By managing files, coordinating visits, and supporting communication between teams, CTAs play a crucial role in keeping day-to-day trial operations organized and audit-ready. Their work helps streamline the complex workflow of clinical research, allowing other professionals to focus on patient safety, data review, and strategic decision-making.
This blog discusses the key roles and responsibilities that are common across every Clinical Trial Assistant job description and define what every CTA does in a clinical research setting.
Who is a Clinical Trial Assistant?
A Clinical Trial Assistant (CTA) is typically an administrative support specialist of the clinical trial who ensures that the trial runs smoothly, on time, and in compliance with regulatory standards. CTAs oversee all kinds of documentation work that keeps the trial organized and compliant. They manage key documents, coordinate site visits, and support communication across teams, allowing other professionals to focus on patient safety, data analysis, and decision-making.
A CTA will primarily work on all the documents required for the trial, from protocols, Case Report Forms (CRFs), and informed consent forms to the Trial Master File (TMF), ensuring that each document is accurate, up-to-date, and compliant with Good Clinical Practice (GCP). They also coordinate site visits, track study progress, and ensure data integrity.
Qualifications & Skills
CTAs typically hold a degree in life sciences, pharmacy, or a related field. Proficiency in tools like EDC systems, CTMS, and Microsoft Office is often preferred. Key skills for success include strong organizational skills, attention to detail, and the ability to manage multiple tasks simultaneously. ccccc
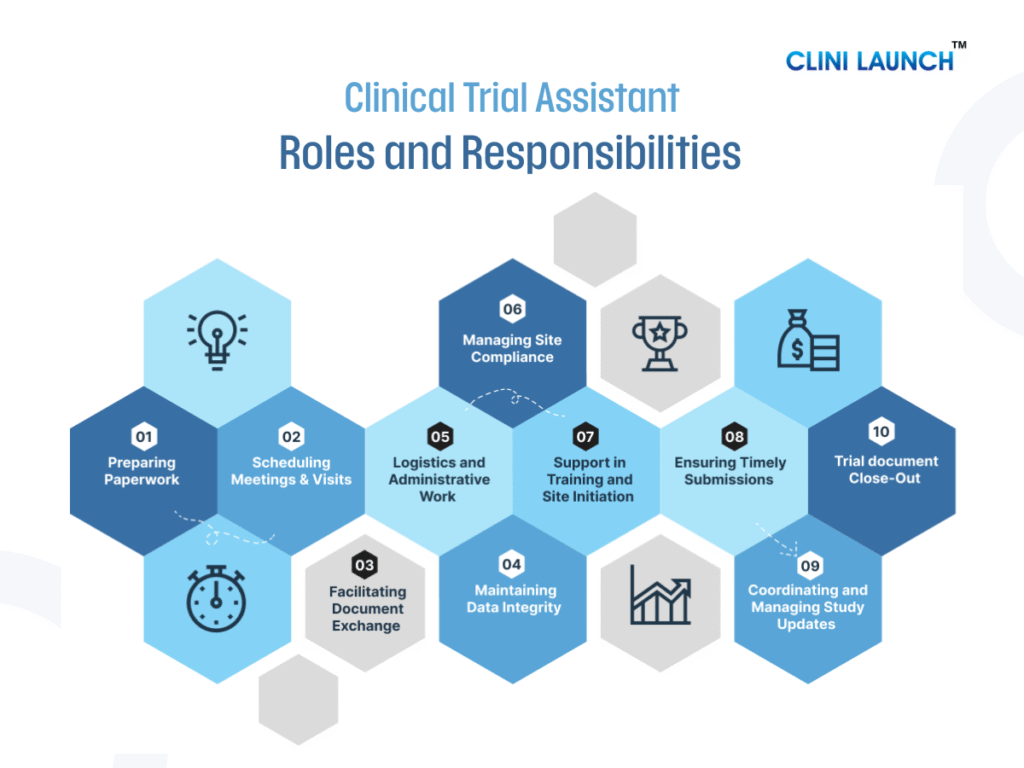
Role and Responsibilities of a Clinical Trial Assistant
1.Preparing Precise Paperwork
Paperwork might sound boring, but those papers hold more value than the entire trial run. Without proper documentation, a clinical trial would have no structure, no flow, and no clear direction to follow.
Clinical Trial Assistants play a crucial role in maintaining this structure. They prepare, organize, and manage essential study documents such as protocols, informed consent forms (ICFs), case report forms (CRFs), investigator brochures, and the Trial Master File (TMF). Every document they handle ensures that the trial moves in a clear, compliant, and traceable manner.
Their job doesn’t stop at filing forms, it’s about maintaining accuracy, version control, and regulatory compliance. Each update must be logged, reviewed, and stored correctly to meet Good Clinical Practice (GCP) and sponsor requirements.
In short, CTAs make sure every document tells the story of the trial, clearly, completely, and correctly. Without their precision in paperwork, even the most advanced study design would fail to run smoothly.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Maintain and manage the Trial Master File (TMF) and electronic TMF (eTMF) using systems such as Veeva Vault Oversee TMF access management and ensure version control Handle maintenance and archival of trial documents throughout the study lifecycle Prepare and maintain trial-related documents and reports for internal and regulatory use Update and manage study-specific trackers to record document status and submissions Stay current with ICH-GCP documentation requirements and quality standards Source: ichgcp careers |
2. Scheduling the Study Meetings and Site Visits
A PI visit, a CTM visit, or a sponsor visit; everything begins with a schedule. Before any stakeholder steps into a site, a CTA ensures that documents are ready, schedules are set, and every essential record is updated and in place. From maintaining investigator site files to preparing meeting trackers, CTAs make sure nothing is missed and every visit runs seamlessly.
Their coordination work involves scheduling meetings, monitoring visits, and team interactions, while ensuring that relevant materials, such as agendas, reports, and visit checklists, are complete and distributed on time. This not only keeps the study inspection-ready but also allows the clinical team to focus on their core responsibilities.
You’ll often see this reflected in job descriptions where CTAs are expected to “provide administrative and project tracking support” or “support clinical research teams with trial documentation and coordination.” These responsibilities require a keen sense of timing, attention to detail, and strong communication skills to ensure that every visit, whether internal or external, happens without a glitch.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Provide administrative and project tracking support to Project Manager(s) and Clinical Trial Manager(s) Support clinical research teams with trial documentation and coordination Maintain accurate and up-to-date study files and records Collate relevant study information and ensure sites are prepared for visits Source: iconplc careers |
3. Facilitating Clinical Research Documents Exchange
A CTA’s office is like a search engine, anyone in the trial can find any relevant document or update they need through them.
Every day, CTAs answer emails, follow up with site coordinators, and update trackers so no request goes unnoticed. If a monitor needs the latest enrollment report, or a sponsor wants to verify document status, the CTA is the first point of contact.
They handle communication between study sites, CROs, vendors, and sponsors, ensuring smooth information exchange across departments. CTAs circulate meeting invites, prepare and distribute minutes, share study updates, and log every correspondence for reference. They also track site activities, subject enrollment, and visit schedules, alerting the team whenever timelines shift or documents need attention.
These are the unseen but essential interactions that keep a trial moving on schedule and in compliance.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Coordinate communications between the research team, sites, vendors, and sponsors Support clinical research teams with trial documentation and correspondence Track site activities and enrollment updates Follow up on pending actions and operational queries Maintain communication logs and trackers for project documentation Source: linkedin |
4. Maintaining Data Integrity and Regulatory Compliance
A document without the exact values can bring chaos to a trial, from the number of participants to study duration, every data point matters. That’s why CTAs play a vital role in ensuring that every entry, report, and record reflects complete accuracy and regulatory alignment.
They are responsible for entering and verifying study data in systems like Electronic Data Capture (EDC) and Clinical Trial Management Systems (CTMS), maintaining version-controlled records that are always audit-ready. CTAs perform quality checks, reconcile discrepancies, and ensure that all study data, whether numerical, procedural, or participant-related, is consistent across documentation.
Beyond data management, they also support the regulatory and compliance side of clinical research. This includes assisting in ethics submissions, maintaining GCP-aligned documentation, and preparing essential files for internal or external audits. By following Standard Operating Procedures (SOPs) and Good Clinical Practice (GCP) guidelines, CTAs make sure the trial remains accurate, compliant, and inspection-ready at all times.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Enter and verify study data in EDC or CTMS systems Conduct quality control checks and reconcile discrepancies Maintain audit-ready data records aligned with GCP Support regulatory submissions and document reconciliation Assist in archiving and inspection preparation for audits Source: syneos health careers |
5. Logistics and Administrative Coordination
The role of a CTA goes beyond paperwork, it’s about keeping the entire trial in motion. From shipping lab kits to tracking invoices, every small coordination task adds up to keeping a clinical study running without a hitch.
CTAs work closely with Project Managers and Clinical Trial Managers to ensure every activity stays on schedule. They handle the coordination of study materials, track their dispatch and receipt, and maintain up-to-date study documentation. On any given day, a CTA might be collating lab shipment reports, following up with vendors, or updating trackers that capture everything from invoice status to site communications.
Their attention to these moving parts makes the trial inspection-ready at all times. The team depends on them to know where every file, form, and shipment stands, because when logistics flow smoothly, the science can too.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Provide administrative and project tracking support to Project Manager(s) and Clinical Trial Manager(s) Coordinate study materials and ensure timely dispatch Collate relevant study information from multiple sources Assist in various clinical trial operations as directed by the research team Maintain and track clinical study documentation and internal reports Source: indeed |
6. Managing Site Compliance and Monitoring
A CTA’s role beyond administrative support includes ensuring study sites remain compliant throughout the trial. From monitoring adherence to study protocols to assisting with site audits, CTAs help identify and address compliance issues early, ensuring the study stays on track and meets regulatory standards.
CTAs track site compliance, ensuring all required documentation is in place and that each site is following the regulatory guidelines. They assist with preparing monitoring reports and site audit reports, identifying non-compliance or protocol deviations, and coordinating corrective actions to maintain study integrity.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Track site compliance with regulatory requirements and study protocols Assist in preparing monitoring and site audit reports Ensure corrective actions are taken to address non-compliance or deviations Source: Iqvia careers |
7. Support in Study Training and Site Initiation
A CTA is also responsible for ensuring that study site staff are properly trained and ready for the trial. From initial site initiation visits to ongoing training, CTAs ensure that all site staff are up-to-date on trial protocols, GCP guidelines, and required documentation practices.
CTAs coordinate and prepare for site initiation visits (SIV) and investigator meetings. They assist in training site staff on the clinical trial’s specific procedures, GCP, and data handling requirements to ensure the study runs smoothly. CTAs may also support investigator recruitment by providing necessary information and answering queries.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Coordinate site initiation visits (SIV) and assist with investigator meetings Train site staff on clinical procedures, GCP, and documentation Assist with investigator recruitment by sharing relevant study information Source: Indeed |
8. Ensuring Timely Trial Documentation Submissions
Timing is everything in clinical trials, and CTAs ensure all documentation is submitted on time. Whether it’s submitting regulatory documents, trial amendments, or ethics committee approvals, CTAs manage the flow of paperwork to ensure no document is missed.
They ensure that all required study documents are submitted to regulatory bodies, sponsors, and ethics committees within specified timelines. CTAs track submissions, ensuring that deadlines are met and documentation is up-to-date. They also review feedback from regulatory bodies and assist with making any necessary corrections to documents.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Ensure timely submissions of required study documents to stakeholders Track submission deadlines and ensure documents are updated accordingly Review feedback from regulatory bodies and make necessary corrections Source: Icon |
9. Coordinating and Managing Study Updates
Effective communication is key to keeping a clinical trial on track. CTAs ensure everyone stays informed. CTAs coordinate updates between the clinical team, study sites, and sponsors, making sure key milestones are communicated to all relevant parties.
They assist with updating the study team on trial progress, milestones, and any changes in the study timeline. CTAs are responsible for tracking study amendments and protocol changes, ensuring these updates are communicated and distributed promptly to all stakeholders, including the clinical team and site staff.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Update the study team on trial progress and key milestones Track and distribute study amendments and protocol updates Ensure timely communication of study changes across stakeholders Source: Iqvia careers |
10. Trial Close-Out and Documentation Archiving
The job isn’t over when the study ends, CTAs also help ensure all documents are archived and properly filed for future reference.
From data reconciliation to document retention, CTAs play a vital role in ensuring the trial’s closure is as organized as its execution. They assist with final reports and study documentation reconciliation at the end of the trial, ensuring all materials are properly archived and stored for future reference or audits. CTAs also help coordinate the return or disposal of clinical trial materials, making sure the study is closed out in full compliance with regulatory guidelines.
| A real JD snippet from a CRO for better understanding: A candidate must be able to: Assist with trial close-out activities such as final documentation reconciliation Ensure proper archival of study materials and trial documents Coordinate return or disposal of clinical trial materials as required Source: Icon |
Daily Routine of a Clinical Trial Assistant:
A Clinical Trial Assistant (CTA) starts their day at the office by logging into their desktop system, checking emails, and reviewing the trial inbox for urgent tasks. As Charlotte Drodge, a CTA, describes, “The day starts with checking the mail, reviewing inboxes, and addressing anything urgent.”
The CTA’s first task may involve data entry, update study files, or preparing reports that need to be sent out. They ensure that all documents, such as Case Report Forms (CRFs), informed consent forms, and the Trial Master File (TMF), are properly organized and accessible. It’s about making sure everything is up-to-date and audit ready.
The CTA coordinates site visits and monitors visits. If a sponsor’s visit is scheduled, the CTA ensures all necessary documentation is prepared and sent in advance. As Charlotte mentions, the role requires great attention to detail: ensuring meeting agendas, visit checklists, and study files are updated and accessible for the visit. The CTA might even accompany the Clinical Research Associates (CRAs) during the site visits to ensure everything runs smoothly.
The CTA also works closely with the data management team, entering study data into systems like EDC and CTMS, ensuring everything is recorded correctly and discrepancies are flagged. If issues arise, they may visit the site to resolve discrepancies directly. As Charlotte explains, much of the work is done remotely, but when needed, CTAs travel to sites to manage the data and documentation directly.
Throughout the month, the CTA helps ensure regulatory submissions are prepared, assists with study progress reports, and makes sure investigator payments are processed. They also manage vendor invoices and ensure the study materials are shipped and received on time. The CTA ensures that milestones are met, study timelines are adhered to, and audits are passed without issue.
Conclusion
A Clinical Trial Assistant (CTA) is more than a support role, as they are the leaders of administrative excellence within clinical operations. Every organized document, scheduled visit, and audit-ready file reflects their precision and ownership. Their ability to coordinate, document, and communicate with accuracy keeps every trial structured, compliant, and progressing on time.
For life-science graduates and professionals aiming to enter the research domain, the CTA role offers a front-row seat to how global studies are planned and executed. It builds the foundation for growth into roles in clinical coordination, project management, and regulatory operations.
Begin your journey with the PG Diploma in Clinical Research at CliniLaunch Research Institute, a globally recognized, IBM-powered program designed to help you master the art of clinical operations and lead with confidence as a future CTA professional.





