Seeking to become the candidate that your pharmacovigilance manager dreams of? If you want to become a pharmacovigilance professional, you are in luck! You have landed on the right page that will help you gain a grip on the top 5 must-know pharmacovigilance interview questions for freshers with answers. At CliniLaunch, we recommend you spend some time and get comfortable with what you might be asked. In this blog, you will be getting must-know interview questions, its scope in India, the best training institute, vacancies, and pharmacovigilance companies in India.
Pharmacovigilance Interview Questions for Freshers
As a fresher, the pharmacovigilance interview you attend, you may always wish to know the interview questions and answers beforehand. You should keep in mind that the interviewer sitting in front is highly experienced and can move a step further. We know there is no list of questions or guides that is perfect for you but what we are bringing to the table is all for your preparation and your better. Therefore, bringing forth a list of a few academic questions for freshers.
What is Pharmacovigilance?
This question is fundamental, typically asked in any pharmacovigilance vacancy and you can define pharmacovigilance using keywords such as adverse drug reactions (ADRs). Take examples from any source. According to the World Health Organization, pharmacovigilance involves the science of assessment, detection, understanding, and prevention of adverse drug reactions or any other drug-related problems.
Difference Between an Adverse Drug Event (ADE) and an Adverse Drug Reaction (ADR)
Let’s focus on adverse drug events only. It is any unwanted and unfavorable occurrence of an event that is temporarily associated with medical product use. In that sense, event occurrence can be a drug, medical device, or vaccination, and “associated with” does not necessarily imply the relation between cause and effect. There exists a subset of undesired occurrences caused by treatment that happened specifically due to medical errors. The error may occur either in prescribing or administration.

Figure 1: Adverse events in a Venn diagram
In comparison, Adverse Drug Reaction refers to dangerous, uncomfortable, and unwanted effects that drugs (including medications) may have. ADRs can be considered as a form of toxicity. However, it is most commonly applied to over-ingestion effects. Although the terms ADR and ADE are quite used in exchange adverse drug reactions are a subset of adverse drug events. The differences can be understood better in the figures below.

Figure 2: ADRs in a Venn diagram of Figure 1
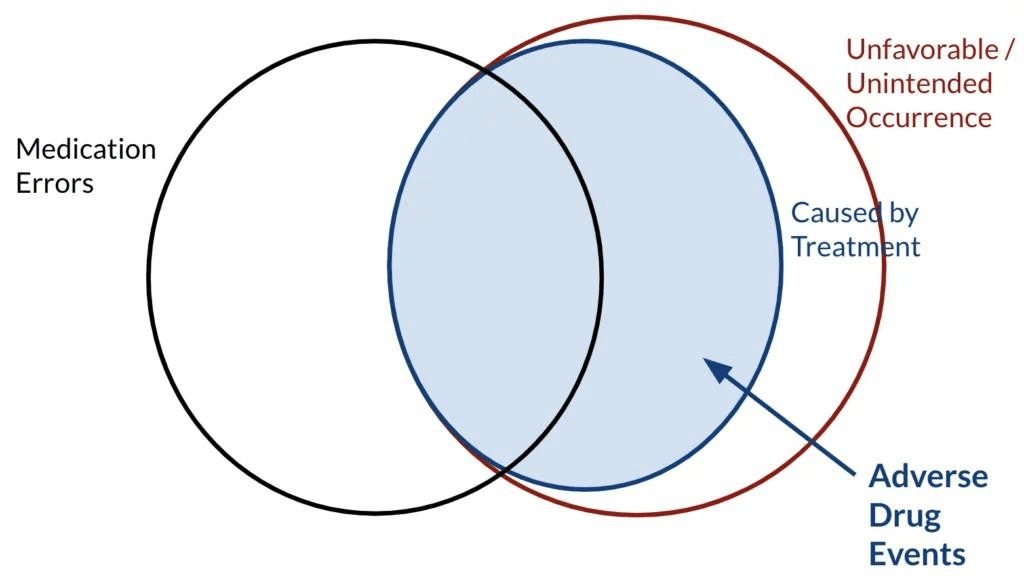
Figure 3: ADEs in a Venn diagram in Figure 1
Comparing the increasing number of medications daily, underreporting of adverse drug events seems to become a pivotal issue. The main objective of pharmacovigilance companies in India is to make as much information as possible available to healthcare professionals to reduce human errors as drug safety is critical as misuse of drugs can cause mortem.
Describe the process for reporting an ADR in India
As a fresher, you may apply for a pharmacovigilance Vacancy, and may get questioned to demonstrate your knowledge of local regulations by mentioning the Central Drugs Standard Control Organization (CDSCO). Demonstrate your knowledge of local regulations by mentioning the Central Drugs Standard Control Organization (CDSCO):
As a pharmacovigilance professional, say, download the Adverse Drug Reaction reporting form from the IPC website, and then fill, scan, and send it to nearby AMC or directly to NCC by post, mail, or fax.
To fill up a valid case report, you should include the mandatory fields, such as:
- Description of the reaction (terms), and reaction data.
- Brand name of the medication
- Your name, address, contact details, qualification, and date of report.
The authorities may share the adverse drug report with other authorities, manufacturers, researchers, or healthcare professionals for further action or investigation.
Pharmacovigilance Scope in India
As a fresher, you can highlight the expanding role of pharmacovigilance in India. According to Future Market Insights Inc., the Indian pharmacovigilance market is expected to increase from USD 6.87 billion to USD 23.32 billion in 2033. The pharmacovigilance scope in the Indian market is anticipated to expand at a compound annual growth rate of 13% during the forecasted period. Due to the development of chronic illness, the demand for pharmacovigilance professionals increased specifically for novel pharmaceuticals created through meticulous clinical research.
How would you approach case processing and reporting in pharmacovigilance?
As a student, you need to demonstrate your understanding of case processing and reporting on pharmacovigilance. Case processing is a fundamental activity providing data for adverse effect analysis, allowing new safety concerns detection, and periodically assessing the benefits-to-risk ratio associated with pharmaceutical product use. The cycle of pharmacovigilance includes adverse events identification followed by its notification, event reporting to the health system using standard operating tools and mechanisms followed by event investigation determining its cause and providing feedback to all its stakeholders.
Launch Your Pharmacovigilance Career with Confidence
By now, you may have strong pharmacovigilance interview questions and understand the importance of this field in India. Equipping yourself with the proper training will put you ahead of the competition and help you land your dream pharmacovigilance vacancy. CliniLaunch is a premier pharmacovigilance training institute offering comprehensive education, training, and certification programs. Our curriculum is specifically designed by industry experts that will equip you with the knowledge and skills to excel in the pharmaceutical field.
Enroll Today for Your Successful Career
Do not wait and think about it.
Visit our website www.clinilaunhcresearch.in now and choose a clinical research course to learn more about our pharmacovigilance training programs.
Take the first step to a rewarding career in the pharmaceutical field.
Contact +91 8904269998.






Good blog
“Such a gem of a post!”
Your article helped me a lot, is there any more related content? Thanks!
Nice article!
Thank you so much for this precious information.