Pharmaceutical industry is booming with companies rapidly expanding their global presence, adopting advanced technologies like AI and precision medicine, and creating new roles that blend science, data, and innovation. The sector now employs over 2.7 million professionals in India, and this number continues to grow each year with increasing investments in research and global market expansion.
The Department of Pharmaceuticals reports that 71% of pharma companies in India hire life science and healthcare graduates, while an Experis survey shows an 28% rise in hiring outlook — nearly 1 in 3 companies expanding their workforce — reflecting the industry’s strong and growing demand for skilled talent.
Thousands of graduates from biotechnology, pharmacy, and healthcare backgrounds are already building successful careers in clinical research, pharmacovigilance, data science, medical writing, and regulatory affairs.
In short, a career in pharma offers rapid growth, global exposure, and a real chance to shape the future of healthcare.
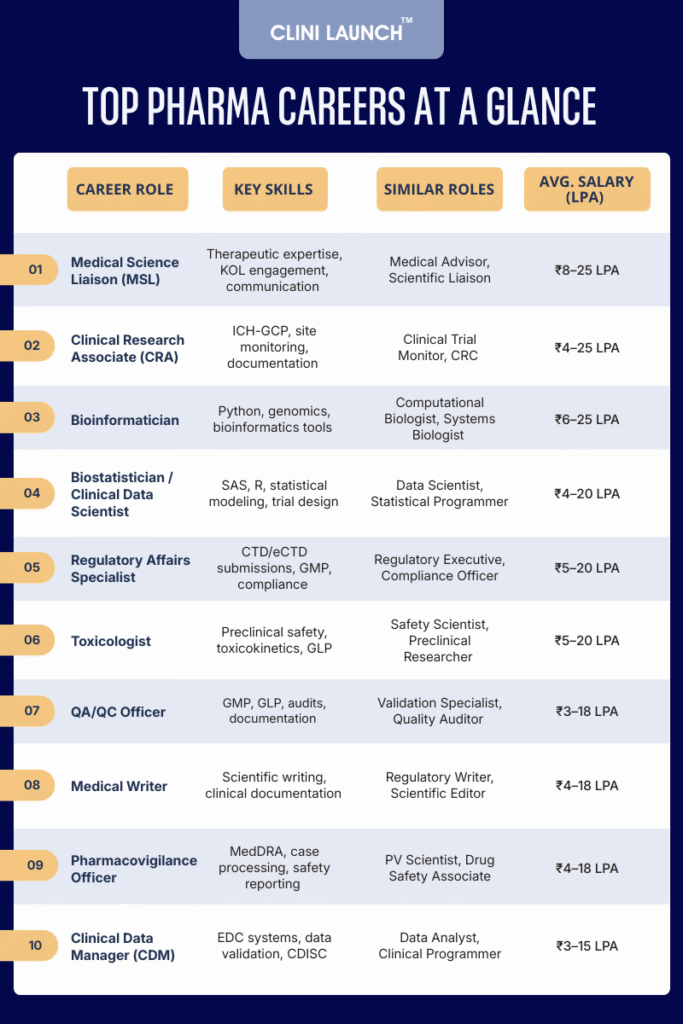
Here are the Top 10 high-paying, in-demand career paths in the pharma industry that are ideal for professionals with life science and healthcare backgrounds.
Top 10 High Paying In-demand Roles in Pharma Industry
1. Medical Science Liaison (MSL)
The MSL role is among the fastest-growing areas in medical affairs, with the global medical affairs outsourcing market expanding at a strong 14–15% CAGR, especially across the Asia-Pacific region, including India. This position sits at the intersection of cutting-edge science and business, demanding professionals with strong scientific knowledge who can bridge the gap between research innovation and clinical application.
About the Role – Medical Science Liaison (MSL)
MSLs share scientific insights with doctors and KOLs, simplifying complex clinical data and supporting evidence-based drug launches. They act as the company’s scientific experts, gathering field insights that guide medical strategy and R&D.
MSLs are therapeutic area experts — often in oncology, immunology, or neurology. IQVIA highlights that MSL serves as the scientific face of the company and their role is “driven by the need to communicate increasingly complex scientific information.”
The role demands peer-level interaction with doctors, researchers, and KOLs, requiring MSLs to interpret data and provide credible insights, with some global positions preferring PhD, MD, or PharmD qualifications.
It’s also among the highest-paying pharmaceutical industry positions, offering starting packages of around ₹4 LPA and scaling up to ₹25 LPA or more, including performance bonuses and business-linked incentives.
Medical Science Liaison (MSL) — Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹10 – ₹18 LPA |
| Senior / Global Level Salary | ₹20 – ₹25 LPA+ in India; up to ₹1.4 Cr/year globally |
| Growth Outlook (Next 5 Years) | +29% salary growth projected; segment growing at 15.6% CAGR |
| Job Mobility | MSLs can shift across therapeutic areas (oncology, neurology, immunology) or transition into strategic roles in global medical affairs, regulatory, or clinical operations |
| Key Benefits | – Salary ceiling with bonuses & incentives, strong work-life balance and travel opportunities, International demand & relocation prospects |
| Why It’s a Top Role | Combines science expertise with communication & leadership, offering global exposure, rapid career growth, and premium pay packages. |
2. Clinical Research Associate (CRA)
The CRA role is one of the most in-demand careers in the clinical research industry, driven by the rapid rise in global and domestic clinical trials. With over 550,000 clinical trials registered worldwide and India emerging as a major hub for research, the need for trained CRAs has grown sharply, yet the supply of skilled professionals remains significantly lower.
About the Role – Clinical Research Associate (CRA)
- A Clinical Research Associate (CRA) monitors trial sites to ensure protocol, ethical, and GCP compliance while verifying accurate patient data and documentation. They coordinate with investigators, resolve site issues, and conduct regular visits to keep trial operations running smoothly.
This field is far from saturation in the pharmaceutical jobs listings. As long as new diseases, new therapies, and new technologies continue to emerge, clinical trials will never stop, making the CRA role essential and future-proof.
CRAs ensure that clinical trials are conducted safely, ethically, and in strict adherence to ICH-GCP guidelines. Because the role demands scientific understanding and regulatory awareness, companies specifically seek candidates from life science or healthcare backgrounds. India currently has far fewer trained CRAs than required, which has led to consistently high demand and competitive salaries.
| Advanced Diploma in Clinical Research | Program Duration: 6 Months |
| Become a job-ready Clinical Research Associate in 6 months with hands-on trial management, monitoring practice, and industry tools. NSDC and Brit Qualis accredited. 8000+ learners enrolled | Live Instructor-led | Skills you’ll build: Clinical Trial Management, Pharmacovigilance, Clinical Data Analysis, Medical Writing, Healthcare Industry Knowledge, Regulatory Compliance, Research Methodology, Regulatory Affairs, Data Management Tools |
The CRA profession is also highly rewarding with salaries typically starting at ₹4–6 LPA, but with monitoring experience professionals quickly progress to ₹10–15 LPA, with senior CRAs and Project Managers earning ₹18–25 LPA or more.
Clinical Research Associate (CRA) — Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹4 – ₹6 LPA for entry-level; ₹7 – ₹10 LPA for experienced CRAs |
| Senior / Global Level Salary | ₹12 – ₹18 LPA in India; USD $80k–$120k internationally |
| Growth Outlook (Next 5 Years) | Clinical research market growing at 8–12% CAGR; India identified as a high-growth trial destination |
| Job Mobility | CRAs can move into Clinical Project Management, Clinical Operations, Quality Assurance, Pharmacovigilance, or Regulatory Affairs |
| Key Benefits | Fast salary growth, Global demand, Strong job stability, Travel and international project exposure |
| Why It’s a Top Role | With trials expanding and decentralized monitoring increasing, demand for skilled CRAs is rising sharply — but the talent shortage remains high. |
3. Bioinformatician
Earlier, bioinformatics roles were limited to a small number of research labs and academic projects. Today, the landscape has transformed—India’s bioinformatics market is growing at a remarkable 18.62% CAGR. With modern drug discovery relying on genomics, NGS, multi-omics, and AI-driven analysis, pharma companies no longer have the luxury of spending years decoding a single gene or validating a target.
About the Role – Bioinformatician
- A Bioinformatician analyzes genetic and molecular data using computational and AI tools. They support drug discovery and precision medicine by identifying targets and driving data-driven research.
Despite the rise of powerful AI/ML tools, the jobs in pharma companies still needs skilled professionals, something that only human expertise can fully deliver. With rising job openings in pharma companies, the demand for skilled bioinformatics talent continues to accelerate.
An entry-level Bioinformatician typically earns ₹4–7 LPA, while experienced specialists and AI-genomics experts can reach ₹15–25 LPA+, making it one of the fastest-growing high-earning careers in pharma.
Bioinformatician — Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹4–7 LPA (entry), ₹8–12 LPA (mid-level) |
| Senior / Global Salary | ₹15–25 LPA+ in India; $90k–$140k globally |
| Growth Outlook | India bioinformatics market growing at 18.6% CAGR |
| Job Mobility | Move into Computational Biology, Genomics, AI/ML in Drug Discovery |
| Key Benefits | High demand, global mobility, strong salaries, cutting-edge work |
| Why It’s a Top Role | Major skill shortage + booming genomics & AI adoption |
| Advanced Diploma in Bioinformatics | Program Duration: 6 Months |
| Become a job-ready Bioinformatics Analyst in 6 months with hands-on experience in genomic/proteomic data analysis, protein modeling, and drug target prediction. IBM-guided labs and industry tools included. 3000+ learners enrolled | Live Instructor-led | Skills you’ll build: Clinical Trial Management, Pharmacovigilance, Clinical Data Analysis, Medical Writing, Healthcare Industry Knowledge, Regulatory Compliance, Research Methodology, Regulatory Affairs, Data Management Tools |
4. Biostatistician / Clinical Data Scientist
A decade ago, biostatistics was a niche role handled mostly by general statisticians. Today, with the rise of large clinical trials, RWE studies, digital health data, and AI-driven research, Biostatisticians have become indispensable. The global biostatistics market is growing at 12%+ CAGR, and India is emerging as a major hub for statistical programming and clinical analytics.
About the Role – Biostatistician
- A Biostatistician designs clinical studies and analyzes trial data using tools like SAS, R, and emerging AI models. They ensure statistical accuracy for regulatory submissions and help drive evidence-based decisions in drug development.
Biostatisticians design studies, analyze trial data, and ensure regulatory-grade evidence for FDA/EMA submissions. As pharma adopts adaptive trials and AI-based modelling, the demand for skilled biostat professionals continues to surge across pharma, CROs, and biotech.
Beginners typically earn ₹4–8 LPA, while experienced specialists with SAS, R, and Bayesian skills can earn ₹15–25 LPA+, making it one of the most stable and future-proof careers in the industry.
Biostatistician / Clinical Data Scientist – Salary, Scope & Benefits Snapshot
| Average Salary (India) | ~ ₹4–8 LPA for entry/mid-level |
| Senior / Global Salary | Senior India roles ~ ₹13–24 LPA on average |
| Growth Outlook | Global consulting services market for biostatistics is projected ~ 9.4% CAGR from 2025–2033. |
| Job Mobility | Can move into roles like Clinical Data Science, RWE Analytics, Statistical Modelling/AI in trials |
| Key Benefits | Strong demand due to expansion of clinical trials & digital health; good compensation; international/remote options |
| Why It’s a Top Role | Skills gap + increasing complexity of trials and data-driven research = high value for biostatisticians |
| Advanced Diploma in Biostatistics | Program Duration: 6 Months |
| Master statistical methods for healthcare research and innovation, learning how to analyze, model, and interpret healthcare data with precision. Through structured modules and guided analysis, gain hands-on experience in probability, sampling, hypothesis testing, correlation, and regression. 5000+ learners enrolled | Live Instructor-led | Skills you’ll build: Probability, Sampling Design, Descriptive Statistics, Inferential Statistics, Regression Analysis, Hypothesis Testing, Analysis of Variance, Data Interpretation, Clinical Trial Design, Healthcare Data Analytics |
5. Regulatory Affairs Specialist
In 2025, the FDA introduced its AI system “Elsa” to speed up clinical and regulatory document review. With AI now entering the approval process, pharma companies urgently need Regulatory Affairs professionals who can manage digital submissions and adapt to fast-changing global guidelines. This shift has sharply increased both the demand and salary growth for skilled RA specialists
About the Role – Regulatory Affairs Specialist
- A Regulatory Affairs Specialist prepares and manages submissions to global agencies and ensures drugs meet all safety and quality standards. They keep companies compliant with evolving regulations and help accelerate safe product approvals.
Regulatory Affairs Specialists ensure that every pharmaceutical product meets all scientific, safety, and legal standards before reaching patients. As drug approvals become more data-driven and globally interconnected, RA professionals guide companies through complex regulations across agencies like the FDA, EMA, MHRA, and CDSCO.
They handle end-to-end compliance—from clinical trial filings and product registration to labeling and post-marketing surveillance. With new AI-enabled standards and evolving global regulations, the role has become more strategic than ever. This rising complexity is pushing salaries higher, with skilled RA professionals now earning ₹8–15 LPA mid-career and ₹15–25 LPA+ in senior roles.
Regulatory Affairs – Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹4–7 LPA (entry), ₹8–15 LPA (mid-level) |
| Senior / Global Salary | ₹15–25 LPA+ in India; ~$80k–$120k globally |
| Growth Outlook | Increasing demand due to evolving regulations & AI-driven compliance |
| Job Mobility | Global RA Submissions, Labeling Specialist, Compliance Manager, RA Lead |
| Key Benefits | High stability, global opportunities, strong pay growth, strategic role |
| Why It’s a Top Role | Constant regulatory changes create continuous demand for skilled professionals |
6. Toxicologist
The global toxicology testing market crossed US $41 billion in 2025, yet the field has only around 9,000 practicing toxicologists in North America and far fewer across emerging markets. Even in India, the toxicity-testing market is projected to reach USD 116.3 million by 2030.
About the Role – Toxicologist
- A Toxicologist evaluates a drug’s safety in preclinical studies and determines safe dosage levels. They ensure compliance with global standards and decide whether a compound can move to human testing.
A Toxicologist is one of the unique pharma roles that plays a crucial role in ensuring that new drugs and chemical compounds are safe for human use. Before any medicine reaches clinical trials, toxicologists conduct preclinical safety testing to study how a compound affects cells, tissues, organs, and biological systems. In India, the average salary for a Toxicologist is around ₹8–9 LPA, while seasoned professionals can earn ₹20 LPA+ as the country’s pharma & safety-testing industry scales rapidly.
Toxicologist – Salary, Scope & Benefits Snapshot
| Average Salary (India) | ₹6–9 LPA (entry), ₹10–18 LPA (mid-level) |
| Senior / Global Salary | ₹20–30 LPA+ in India; ~$90k–$150k globally |
| Growth Outlook | India’s early toxicity testing market projected to grow from USD 49.3M (2024) to USD 116.3M (2030) |
| Job Mobility | Preclinical Scientist, Safety Assessor, Toxicology Lead, Regulatory Toxicologist |
| Key Benefits | High demand, strong global relevance, essential for drug safety, recession-proof |
| Why It’s a Top Role | Severe shortage of trained toxicologists + rising R&D and safety-testing requirements |
7. Quality Assurance / Quality Control (QA/QC) Officer
Despite being one of the most essential career in pharma, many students and job-seekers are unaware of how crucial QA/QC roles are in drug development and manufacturing. This lack of awareness has created a noticeable talent gap—even as the pharmaceutical quality-testing market continues to expand rapidly.
As a result, jobs in pharma companies require skilled QA/QC professionals who understand GMP, documentation practices, audits, and compliance — making this one of the most in-demand and high-growth career paths in the industry today. With increasing pharmaceutical job openings, QA/QC Officers act as the final guardians of product quality, earning ₹3.5–10 LPA with salaries rising sharply with experience.
About the Role – QA/QC Officer
- A QA/QC Officer ensures GMP/GLP/GCP compliance through audits and quality checks. They act as the final gate to ensure products meet required standards.
Quality Assurance / Quality Control (QA/QC) Officer – Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹3.5–5.5 LPA (entry), ₹6–10 LPA (mid-level) |
| Senior / Global Salary | ₹12–20 LPA+ in India; ~$70k–$110k globally |
| Growth Outlook | Rising demand due to increased GMP audits, regulatory scrutiny, and expanding pharma manufacturing |
| Job Mobility | QA Specialist, QC Analyst, IPQA Officer, Quality Manager, GMP Auditor |
| Key Benefits | High job stability, strong industry demand, essential regulatory role |
| Why It’s a Top Role | Talent shortage + rising quality compliance standards across Indian and global pharma |
8. Medical Writer
Post-COVID, medical writing has grown rapidly as clinical research and digital scientific communication expanded — with many companies shifting documentation work to remote models. The global medical writing market is projected to grow to USD 10.26 billion by 2032, making this one of the fastest-growing and increasingly remote-friendly careers in life sciences.
About the Role – Medical Writer
- A Medical Writer converts clinical data into clear, accurate documents and publications. They ensure scientific accuracy while supporting clinical, regulatory, and research teams.
Medical Writing career in pharma involves converting complex clinical and scientific data into clear, accurate documents, and with rising trials and stricter regulations, their demand has grown rapidly across India and globally — making it a strong pharmaceutical career choice. They typically earn ₹4–7 LPA at entry level and ₹8–14 LPA+ as they gain experience.
Medical Writer – Salary, Scope & Benefits Snapshot
| Average Salary (India) | ₹4–7 LPA (entry), ₹8–14 LPA (mid-level) |
| Senior / Global Salary | ₹15–25 LPA+ in India; ~$70k–$120k globally |
| Growth Outlook | Rapid growth due to increased clinical trials, digital health content, and global outsourcing |
| Job Mobility | Scientific Writer, Regulatory Writer, Publication Specialist, Medical Communication Lead |
| Key Benefits | High demand, remote-friendly, global clients, strong salary progression |
| Why It’s a Top Role | Fast expansion of medical communication + shortage of skilled scientific writers |
9. Pharmacovigilance Officer / Drug Safety Associate
When graduates or career-switchers look to enter the pharmaceutical industry, Pharmacovigilance is often the first role everyone talks about. It has become one of the most accessible and widely chosen entry points into pharma, supported by rising demand, expanding safety requirements, and a surge in dedicated PV training programs across India.
About the Role – Pharmacovigilance Officer
- A Pharmacovigilance Officer processes adverse event reports and ensures timely global safety reporting. They help maintain drug safety during trials and post-marketing.
Pharmacovigilance (PV) Officers—also called Drug Safety Associates—play a key role in monitoring, detecting, assessing, and preventing adverse drug reactions (ADRs). Their work ensures that medicines remain safe throughout clinical trials and even long after they reach the market. Pharmacovigilance career in Pharma has become the most talked-about transition role in pharma, with growing demand and starting salaries around ₹3.5–5.5 LPA.
Pharmacovigilance – Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹3.5–5.5 LPA (entry), ₹6–10 LPA (mid-level) |
| Senior / Global Salary | ₹12–18 LPA+ in India; ~$60k–$100k globally |
| Growth Outlook | Global PV market growing strongly due to rising ADR reporting and regulatory scrutiny |
| Job Mobility | PV Specialist, Aggregate Reporting, Signal Detection Analyst, Safety Scientist |
| Key Benefits | High stability, strong global demand, clear career progression |
| Why It’s a Top Role | Increasing clinical trials + stricter safety monitoring = continuous hiring need |
| Advanced Diploma AI Integration in Drug Safety and Compliance | Program Duration: 6 Months |
| Become a skilled professional in AI-powered Drug Safety and Pharmacovigilance with practical experience in regulatory affairs, medical writing, and AI-driven tools. Learn to integrate AI for automated ADR detection, signal management, and compliance reporting. 3000+ learners enrolled | Live Instructor-led | Skills you’ll build: Pharmacovigilance, ADR Signal Detection, Risk Prediction, MedDRA Coding, WHO Drug Dictionary, Regulatory Affairs (FDA, EMA, CDSCO), Medical Writing, ICSR Reporting, AI in Regulatory Compliance, Natural Language Processing (NLP), Machine Learning for Drug Safety, AI Automation in Documentation, Compliance Audits, AI Ethics, Data Privacy, IBM Watson AI Services, Cloud-based AI Tools. |
10. Clinical Data Manager (CDM)
Clinical Data Management was once seen as a “numbers and paperwork” job that many graduates ignored — but today it has become one of the highest-demand and fastest-growing roles in clinical research, driven by digital trials, rising data volumes, and the need for accurate, high-quality clinical data.
About the Role – Clinical Data Manager
- A Clinical Data Manager designs and manages EDC systems, reviews and cleans trial data, and ensures CDISC/GCP compliance. They handle queries, lock databases, and prepare reliable datasets for statistical analysis.
A Clinical Data Manager ensures that the data collected during clinical trials is accurate, consistent, and reliable. They oversee the entire data lifecycle—from designing electronic data capture (EDC) systems to reviewing, cleaning, validating, and locking the database for statistical analysis.
Their work determines whether trial results are trustworthy and whether a drug can move to the next stage of development. As trials grow in complexity, the role has become central to clinical operations and regulatory success, with salaries typically ranging from ₹4–7 LPA for entry-level roles and ₹8–14 LPA+ for mid-level professionals.
Clinical Data Manager – Salary, Scope & Benefits Snapshot
| Category | Details |
| Average Salary (India) | ₹4–7 LPA (entry), ₹8–14 LPA (mid-level) |
| Senior / Global Salary | ₹15–25 LPA+ in India; ~$80k–$130k globally |
| Growth Outlook | High demand due to digital trials, EDC adoption, and rising global clinical research |
| Job Mobility | Data Analyst, Lead Data Manager, CDISC Specialist, Clinical Data Scientist |
| Key Benefits | Strong demand, high salaries, global exposure, tech-driven role |
| Why It’s a Top Role | Massive data volumes + digital transformation = continuous hiring for CDM experts |
Conclusion
At CliniLaunch Research Institute, we offer specialized programs and certifications that prepare you for these pharma roles — from clinical research and pharmacovigilance to data science and regulatory affairs.
Explore our website to learn more and take the next step toward your successful career in pharma sector.
Visit CliniLaunch Research Institute and start your career journey today.
`





